A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol
- PMID: 33538150
- PMCID: PMC8252956
- DOI: 10.3324/haematol.2020.260935
A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol
Abstract
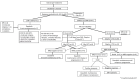
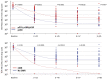
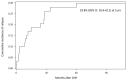
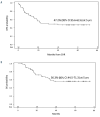
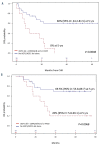
The GIMEMA LAL1509 protocol, designed for adult (≥18-60 years) de novo Ph+ acute lymphoblastic leukemia patients, was based on a dasatinib plus steroids induction - with central nervous system prophylaxis - followed by dasatinib alone in patients in complete molecular response or chemotherapy and/or allogeneic transplantation in patients not reaching a complete molecular response. Sixty patients (median age 41.9 years) were enrolled: 33 were p190+, 18 p210+ and 9 p190/p210+. At the end of induction (day +85), 58 patients (97%) achieved a complete hematologic remission. No deaths in induction were recorded. Eleven patients (18.3%) obtained a complete molecular response. Among non-complete molecular responders (n=47), 22 underwent an allogeneic transplant. Seventeen hematologic relapses occurred (median 7 months, range 3-40.1), 13 during consolidation and 4 post-transplant. ABL1 mutations (5 T315I, 3 V299L, 1 E281K and 1 G254E) were found in 10/13 relapsed cases. With a median follow-up of 57.4 months (range: 4.2-75.6), overall survival and disease-free survival are 56.3% and 47.2%. A better diseasefree survival was observed in patients who obtained a molecular response at day +85 compared to cases who did not. The presence of additional copy number aberrations - IKZF1 plus CDKN2A/B and/or PAX5 deletions - was the most important unfavorable prognostic factor on overall and disease-free survival (p=0.005 and p=0.0008). This study shows that in adult Ph+ ALL long-term survivals can be achieved with a total-therapy strategy based on a chemo-free induction and, in complete molecular responders, also without further systemic chemotherapy. Finally, the screening of additional copy number aberrations should be included in the diagnostic work-up. EudraCT 2010-019119-39.
Figures

Comment in
-
A chemotherapy-free regimen for Philadelphia chromosome-positive acute lymphoblastic leukemia: are we there yet?Haematologica. 2021 Jul 1;106(7):1781-1782. doi: 10.3324/haematol.2020.278077. Haematologica. 2021. PMID: 33538156 Free PMC article. No abstract available.
References
-
- Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85-109. - PubMed
-
- Mancini M, Scappaticci D, Cimino G, et al. . A comprehensive genetic classification of adult acute lymphoblastic leukemia (ALL): analysis of the GIMEMA 0496 protocol. Blood. 2005;105(9):3434-3441. - PubMed
-
- Burmeister T, Schwartz S, Bartram CR, et al. . Patients’ age and BCR–ABL frequency in adult B- precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008;112(3):918-919. - PubMed
-
- Yanada M, Takeuchi J, Sugiura I, et al. . High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCRABL- positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460-466. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

