The right immune-modulation at the right time: thymosin α1 for prevention of severe COVID-19 in cancer patients
- PMID: 33538178
- PMCID: PMC7874885
- DOI: 10.2217/fon-2020-0754
The right immune-modulation at the right time: thymosin α1 for prevention of severe COVID-19 in cancer patients
Abstract
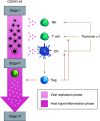
We presented the rationale for the use of thymosin α1 as prophylaxis of severe COVID-19 in cancer patients undergoing active treatment, constituting the background for the PROTHYMOS study, a prospective, multicenter, open-label, Phase II randomized study, currently in its start-up phase (Eudract no. 2020-006020-13). We aim to offer new hope for this incurable disease, especially to frail patient population, such as patients with cancer. The hypothesis of an effective prophylactic approach to COVID-19 would have immediate clinical relevance, especially given the lack of curative approaches. Moreover, in the 'COVID-19 vaccine race era' both clinical and biological results coming from the PROTHYMOS trials could even support the rationale for future combinatorial approaches, trying to rise vaccine efficacy in frail individuals.
Keywords: COVID-19; SARS-CoV-2; cancer; cancer patients; prevention; prophylaxis; vaccination.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
