Evidence-based clinical practice guidelines for irritable bowel syndrome 2020
- PMID: 33538894
- PMCID: PMC7932982
- DOI: 10.1007/s00535-020-01746-z
Evidence-based clinical practice guidelines for irritable bowel syndrome 2020
Erratum in
-
Correction: Evidence-based clinical practice guidelines for irritable bowel syndrome 2020.J Gastroenterol. 2023 Nov;58(11):1165. doi: 10.1007/s00535-023-02044-0. J Gastroenterol. 2023. PMID: 37752291 Free PMC article. No abstract available.
Abstract
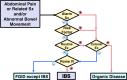
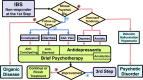
Managing irritable bowel syndrome (IBS) has attracted international attention because single-agent therapy rarely relieves bothersome symptoms for all patients. The Japanese Society of Gastroenterology (JSGE) published the first edition of evidence-based clinical practice guidelines for IBS in 2015. Much more evidence has accumulated since then, and new pharmacological agents and non-pharmacological methods have been developed. Here, we report the second edition of the JSGE-IBS guidelines comprising 41 questions including 12 background questions on epidemiology, pathophysiology, and diagnostic criteria, 26 clinical questions on diagnosis and treatment, and 3 questions on future research. For each question, statements with or without recommendations and/or evidence level are given and updated diagnostic and therapeutic algorithms are provided based on new evidence. Algorithms for diagnosis are requisite for patients with chronic abdominal pain or associated symptoms and/or abnormal bowel movement. Colonoscopy is indicated for patients with one or more alarm symptoms/signs, risk factors, and/or abnormal routine examination results. The diagnosis is based on the Rome IV criteria. Step 1 therapy consists of diet therapy, behavioral modification, and gut-targeted pharmacotherapy for 4 weeks. For non-responders, management proceeds to step 2 therapy, which includes a combination of different mechanistic gut-targeted agents and/or psychopharmacological agents and basic psychotherapy for 4 weeks. Step 3 therapy is for non-responders to step 2 and comprises a combination of gut-targeted pharmacotherapy, psychopharmacological treatments, and/or specific psychotherapy. These updated JSGE-IBS guidelines present best practice strategies for IBS patients in Japan and we believe these core strategies can be useful for IBS diagnosis and treatment globally.
Keywords: 5-HT3 antagonists; 5-HT4 agonists; Antibiotics; Antidepressant; Brain-gut interactions; Complications; Diagnosis; Epidemiology; Functional bowel disorder (FBD); Functional gastrointestinal disorders (FGIDs); Infection; Inflammation; Intestinal secretagogues (gut epithelial modifier); Irritable bowel syndrome (IBS); Microbiota; Mucosal permeability; Pathophysiology; Probiotics; Prognosis; Psychosocial stress; Psychotherapy; Rome IV criteria; Treatment.
Conflict of interest statement
Any financial relationship with enterprises, businesses or academic institutions in the subject matter or materials discussed in the manuscript are listed as follows: 1) those from which the authors, the spouse, partner or immediate relatives of the authors have received individually any income, honoraria or any other type of renumeration; Astellas Pharmaceutical, EA Pharma, Mylan EPD, Takeda Pharmaceutical, Mochida Pharmaceutical, Otsuka Pharmaceutical, Miyarisan Pharmaceutical, and 2) those from which the academic institutions of the authors received support (commercial/academic cooperation): Astellas Pharmaceutical, EA Pharma, Zespri International Japan, Tsumura, Biofermin, Taiyo Kagaku.
Figures


References
-
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Imren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.014. - DOI - PubMed
-
- Fukudo S, Kaneko H, Akiho H, Inamori M, Endo Y, Okumura T, Kanazawa M, Kamiya T, Sato K, Chiba T, Furuta K, Yamato S, Arakawa T, Fujiyama Y, Azuma T, Fujimoto K, Mine T, Miura S, Kinoshita Y, Sugano K, Shimosegawa T. Evidence-based clinical practice guidelines for irritable bowel syndrome. J Gastroenterol. 2015;50:11–30. doi: 10.1007/s00535-014-1017-0. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

