Adverse Drug Reactions from Real-World Data in Inflammatory Bowel Disease Patients in the IBDREAM Registry
- PMID: 33538994
- PMCID: PMC8053178
- DOI: 10.1007/s40264-021-01045-3
Adverse Drug Reactions from Real-World Data in Inflammatory Bowel Disease Patients in the IBDREAM Registry
Abstract
Introduction: Inflammatory bowel disease (IBD) frequently requires chronic immunosuppressive treatment and active involvement from patients during treatment decision making. Information about the risk of developing adverse drug reactions (ADRs) to IBD therapies is required in this process.
Objective: The aim of this study was to describe the ADRs reported in IBD patients from real-world data, using the Dutch nationwide IBDREAM registry, and compare the occurrence and cumulative incidences with the Summary of Product Characteristics (SmPC) of the associated drugs.
Methods: In this retrospective multicentre study, ADRs related to IBD medication were assessed. Only reports associated with the use of drugs used for the maintenance treatment of IBD were included. All ADRs were verified by healthcare professionals and coded by trained pharmacovigilance assessors.
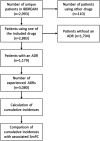
Results: In total, 3080 ADRs were reported in 1179 patients. Twenty-three new drug-ADR associations related to the use of azathioprine, mercaptopurine, infliximab, oral mesalamine and thioguanine were reported in the IBDREAM registry that were not mentioned in the corresponding SmPCs. The most frequently reported new association was pyrexia for azathioprine (3.1%) and mercaptopurine (4.9%). In addition, there were seven ADRs with a higher cumulative incidence in IBDREAM compared with the SmPC, and included, among others, arthralgia during mercaptopurine use (2.5%), and diarrhoea (1.4%), alopecia (1.2%) and infections (1.6%) during azathioprine use.
Conclusions: Based on real-world data, ADR reporting demonstrated new ADRs and higher incidences of ADRs to IBD therapies. This information will contribute to drug safety by updating the SmPCs, allowing better risk assessment and communication towards patients.
Conflict of interest statement
Elaine L. Giraud, Pepijn W.A. Thomas, Jette A. van Lint, Eugene P. van Puijenbroek, Tessa E.H. Römkens, Maurice G.V.M. Russel, Jeroen M. Jansen, Naomi T. Jessurun, and Frank Hoentjen declare they have no conflicts of interest. Rachel L. West reports that she has participated in advisory boards and/or received financial compensation from Janssen and Pfizer, all outside the submitted work.
Figures
References
-
- A Guideline on Summary of Product Characteristics (SmPC). Directorate-General. European Commission Enterprise and Industry; 2009. p. 29.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials