KLF5 Is Induced by FOXO1 and Causes Oxidative Stress and Diabetic Cardiomyopathy
- PMID: 33539225
- PMCID: PMC7870005
- DOI: 10.1161/CIRCRESAHA.120.316738
KLF5 Is Induced by FOXO1 and Causes Oxidative Stress and Diabetic Cardiomyopathy
Abstract
Rationale: Diabetic cardiomyopathy (DbCM) is a major complication in type-1 diabetes, accompanied by altered cardiac energetics, impaired mitochondrial function, and oxidative stress. Previous studies indicate that type-1 diabetes is associated with increased cardiac expression of KLF5 (Krüppel-like factor-5) and PPARα (peroxisome proliferator-activated receptor) that regulate cardiac lipid metabolism.
Objective: In this study, we investigated the involvement of KLF5 in DbCM and its transcriptional regulation.
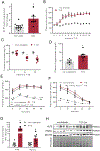
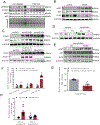
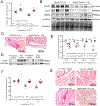
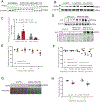
Methods and results: KLF5 mRNA levels were assessed in isolated cardiomyocytes from cardiovascular patients with diabetes and were higher compared with nondiabetic individuals. Analyses in human cells and diabetic mice with cardiomyocyte-specific FOXO1 (Forkhead box protein O1) deletion showed that FOXO1 bound directly on the KLF5 promoter and increased KLF5 expression. Diabetic mice with cardiomyocyte-specific FOXO1 deletion had lower cardiac KLF5 expression and were protected from DbCM. Genetic, pharmacological gain and loss of KLF5 function approaches and AAV (adeno-associated virus)-mediated Klf5 delivery in mice showed that KLF5 induces DbCM. Accordingly, the protective effect of cardiomyocyte FOXO1 ablation in DbCM was abolished when KLF5 expression was rescued. Similarly, constitutive cardiomyocyte-specific KLF5 overexpression caused cardiac dysfunction. KLF5 caused oxidative stress via direct binding on NADPH oxidase (NOX)4 promoter and induction of NOX4 (NADPH oxidase 4) expression. This was accompanied by accumulation of cardiac ceramides. Pharmacological or genetic KLF5 inhibition alleviated superoxide formation, prevented ceramide accumulation, and improved cardiac function in diabetic mice.
Conclusions: Diabetes-mediated activation of cardiomyocyte FOXO1 increases KLF5 expression, which stimulates NOX4 expression, ceramide accumulation, and causes DbCM.
Keywords: NADPH oxidases; ceramides; diabetic cardiomyopathy; forkhead box protein O1; oxidative stress; peroxisome proliferator-activated receptors.
Conflict of interest statement
DISCLOSURES
MCS has patents No. US 10,045,951 B2, No. US 10,030,040 B2, and No. US 9,987,321 B2 issued, patents No. PCT/US2016/049780, No. PCT/US17/35960, and No. PCT/US2008/006694 pending, and has a founder’s equity position in LignaMed, LLC. All other authors have no competing interests or conflicts to report.
Figures



Comment in
-
Heart Failure in Diabetes: Still a Vexing Problem.Circ Res. 2021 Feb 5;128(3):358-359. doi: 10.1161/CIRCRESAHA.121.318670. Epub 2021 Feb 4. Circ Res. 2021. PMID: 33539222 No abstract available.
References
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. Idf diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50 - PubMed
-
- Lee MMY, McMurray JJV, Lorenzo-Almoros A, Kristensen SL, Sattar N, Jhund PS, Petrie MC. Diabetic cardiomyopathy. Heart. 2018 - PubMed
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

