Framework for the treatment and reporting of missing data in observational studies: The Treatment And Reporting of Missing data in Observational Studies framework
- PMID: 33539930
- PMCID: PMC8168830
- DOI: 10.1016/j.jclinepi.2021.01.008
Framework for the treatment and reporting of missing data in observational studies: The Treatment And Reporting of Missing data in Observational Studies framework
Abstract
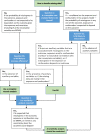
Missing data are ubiquitous in medical research. Although there is increasing guidance on how to handle missing data, practice is changing slowly and misapprehensions abound, particularly in observational research. Importantly, the lack of transparency around methodological decisions is threatening the validity and reproducibility of modern research. We present a practical framework for handling and reporting the analysis of incomplete data in observational studies, which we illustrate using a case study from the Avon Longitudinal Study of Parents and Children. The framework consists of three steps: 1) Develop an analysis plan specifying the analysis model and how missing data are going to be addressed. An important consideration is whether a complete records' analysis is likely to be valid, whether multiple imputation or an alternative approach is likely to offer benefits and whether a sensitivity analysis regarding the missingness mechanism is required; 2) Examine the data, checking the methods outlined in the analysis plan are appropriate, and conduct the preplanned analysis; and 3) Report the results, including a description of the missing data, details on how the missing data were addressed, and the results from all analyses, interpreted in light of the missing data and the clinical relevance. This framework seeks to support researchers in thinking systematically about missing data and transparently reporting the potential effect on the study results, therefore increasing the confidence in and reproducibility of research findings.
Keywords: ALSPAC; Missing data; Multiple imputation; Observational studies; Reporting; STRATOS initiative.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Hogan J.W., Roy J., Krokontzelou C. Tutorial in biostatistics: handling drop-out in longitudinal studies. Stat Med. 2004;23:1455–1497. - PubMed
-
- Kalaycioglu O., Copas A., King M., Omar R.Z. A comparison of multiple imputation methods for handling missing data in repeated measurements observational studies. J R Stat Soc Ser A. 2016;179:683–706.
Publication types
MeSH terms
Grants and funding
- MC_UU_12023/21/MRC_/Medical Research Council/United Kingdom
- MC_UU_00004/07/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/29/MRC_/Medical Research Council/United Kingdom
- MC UU 12023/21/MRC_/Medical Research Council/United Kingdom
- MC UU 12023/29/MRC_/Medical Research Council/United Kingdom
- MC_PC_15018/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/6/MRC_/Medical Research Council/United Kingdom
- MR/L012081/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- P30 AI042853/AI/NIAID NIH HHS/United States
- 102215/2/13/2/WT_/Wellcome Trust/United Kingdom
- G9815508/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/3/MRC_/Medical Research Council/United Kingdom
- MC_PC_19009/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

