Smoking Behavior in Patients With Early-Stage NSCLC: A Report From ECOG-ACRIN 1505 Trial
- PMID: 33539971
- PMCID: PMC8383783
- DOI: 10.1016/j.jtho.2020.12.017
Smoking Behavior in Patients With Early-Stage NSCLC: A Report From ECOG-ACRIN 1505 Trial
Abstract
Introduction: Smoking cessation has been reported to benefit patients even after a diagnosis of lung cancer. We studied the smoking behavior of patients who participated in a phase 3 trial of adjuvant therapy following resection of stages IB-IIIA NSCLC.
Methods: The ECOG-ACRIN 1505 was conducted to determine whether the addition of bevacizumab to adjuvant chemotherapy would improve overall survival (OS) for patients with early-stage NSCLC. Studying the association between smoking status and OS was a secondary end point. Patients completed a questionnaire on their smoking habits at baseline, 3, 6, 9, and 12 months.
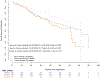
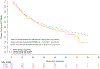
Results: A total of 1501 patients were enrolled, and 99.8%, 95%, 94%, 93%, and 93% responded to the questionnaire at baseline, 3, 6, 9, and 12 months, respectively. A total of 90% reported a current or previous history of cigarette smoking. In addition, 60% of nonsmokers at enrollment reported smoking after diagnosis (before randomization); however, 1% of them reported smoking at 12 months. Furthermore, 94% of the respondents smoked none/fewer cigarettes daily at 12 months. The incidence of grades 3-5 toxicity on treatment was 68%, 76%, and 72% in never, former, and current smokers, respectively (p = 0.05). The disease-free survival for never-smokers relative to current and former smokers was (hazard ratio [HR] 0.93, p = 0.64 and HR 1.05, p = 0.72), and OS was (adjusted HR for death 0.54, p = 0.005 and adjusted HR for death 0.68, p = 0.03), respectively.
Conclusions: This is the first comprehensive, prospective report of smoking habits in patients with NSCLC patients from a phase III early-stage trial. There was a high rate of smoking reduction and cessation following study entry. The disease-free survival did not differ significantly between smokers and never smokers, though there were less grade 3-5 toxicities and more favorable OS in never-smokers.
Keywords: Chemotherapy; Clinical trial; Lung cancer; Outcomes; Smoking; Tobacco cessation.
Copyright © 2021 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement:
Dr Gandara reports Consultant and honorarium from Roche-Genentech.
Dr Steuer reports advisory board honorarium from Abbvie, Lilly, Bergen Bio, Armo
Dr Wakelee reports grants form Gilead, Personal Fees from AztraZeneca, Xcovery, Janssen, Daiichi Sankyo, Helsinn, Mirat
Dr. Ramalingam reports grants and other from Amgen, other from Abbvie, grants and other from Astra Zeneca, grants and other from BMS, other from Genentech, other from Roche, grants and other from Merck, grants and other from Takeda, grants from Tesaro, grants from Advaxis
The other Authors report no relevant COI
Figures
Comment in
-
Critical Determinants of Cancer Treatment Outcomes: Smoking Must Be Addressed at the Highest Levels in Cancer Care.J Thorac Oncol. 2021 Jun;16(6):891-893. doi: 10.1016/j.jtho.2021.03.010. Epub 2021 Apr 22. J Thorac Oncol. 2021. PMID: 33895106 No abstract available.
References
-
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3: 733–744. - PubMed
-
- National Center for Chronic Disease P, Health Promotion Office on S, Health. Reports of the Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US), 2014.
-
- Baser S, Shannon VR, Eapen GA, et al. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest. 2006;130: 1784–1790. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 CA093373/CA/NCI NIH HHS/United States
- UG1 CA189974/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- UG1 CA233340/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233341/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- UG1 CA233247/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical