An Androsterone-H2 @C60 hybrid: Synthesis, Properties and Molecular Docking Simulations with SARS-Cov-2
- PMID: 33540487
- PMCID: PMC8014820
- DOI: 10.1002/cplu.202000770
An Androsterone-H2 @C60 hybrid: Synthesis, Properties and Molecular Docking Simulations with SARS-Cov-2
Abstract
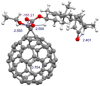
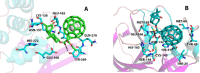
We report the synthesis and characterization of a fullerene-steroid hybrid that contains H2 @C60 and a dehydroepiandrosterone moiety synthesized by a cyclopropanation reaction with 76 % yield. Theoretical calculations at the DFT-D3(BJ)/PBE 6-311G(d,p) level predict the most stable conformation and that the saturation of a double bond is the main factor causing the upfield shielding of the signal appearing at -3.13 ppm, which corresponds to the H2 located inside the fullerene cage. Relevant stereoelectronic parameters were also investigated and reinforce the idea that electronic interactions must be considered to develop studies on chemical-biological interactions. A molecular docking simulation predicted that the binding energy values for the protease-hybrid complexes were -9.9 kcal/mol and -13.5 kcal/mol for PLpro and 3CLpro respectively, indicating the potential use of the synthesized steroid-H2 @C60 as anti-SARS-Cov-2 agent.
Keywords: SARS-Cov-2; cyclopropanation; fullerenes; molecular docking; steroid hybrids.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kurotobi K., Murata Y., Science 2011, 333, 613–616. - PubMed
-
- None
-
- Beyers R., Kiang C.-H., Johnson R. D., Salem J. R., De Vries M. S., Yannoni C. S., Bethune D. S., Dorn H. C., Burbank P., Harichi K., Stevenson S., Nature 1994, 370, 196–199;
-
- Chaur M. N., Melin F., Ortíz A. L., Echegoyen L., Angew. Chem. Int. Ed. 2009, 48, 7514–7538; - PubMed
- Angew. Chem. 2009, 121, 7650–7675.
-
- Stevenson S., Rice G., Glass T., Harich K., Cromer F., Jordan M. R., Craft J., Hadjun E., Bible R., Olmstead M. M., Maitra K., Fischer A. J., Balch A. L., Dorn H. C., Nature 1999, 401, 55–57.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

