Sulfatase 2 Is Associated with Steroid Resistance in Childhood Nephrotic Syndrome
- PMID: 33540508
- PMCID: PMC7867139
- DOI: 10.3390/jcm10030523
Sulfatase 2 Is Associated with Steroid Resistance in Childhood Nephrotic Syndrome
Abstract
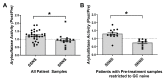
Glucocorticoid (GC) resistance complicates the treatment of ~10-20% of children with nephrotic syndrome (NS), yet the molecular basis for resistance remains unclear. We used RNAseq analysis and in silico algorithm-based approaches on peripheral blood leukocytes from 12 children both at initial NS presentation and after ~7 weeks of GC therapy to identify a 12-gene panel able to differentiate steroid resistant NS (SRNS) from steroid-sensitive NS (SSNS). Among this panel, subsequent validation and analyses of one biologically relevant candidate, sulfatase 2 (SULF2), in up to a total of 66 children, revealed that both SULF2 leukocyte expression and plasma arylsulfatase activity Post/Pre therapy ratios were greater in SSNS vs. SRNS. However, neither plasma SULF2 endosulfatase activity (measured by VEGF binding activity) nor plasma VEGF levels, distinguished SSNS from SRNS, despite VEGF's reported role as a downstream mediator of SULF2's effects in glomeruli. Experimental studies of NS-related injury in both rat glomeruli and cultured podocytes also revealed decreased SULF2 expression, which were partially reversible by GC treatment of podocytes. These findings together suggest that SULF2 levels and activity are associated with GC resistance in NS, and that SULF2 may play a protective role in NS via the modulation of downstream mediators distinct from VEGF.
Keywords: FSGS; glucocorticoids; steroid resistant nephrotic syndrome; sulfatase 2; vascular endothelial growth factor (VEGF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



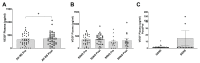

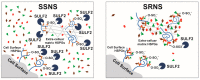
 , SULF2, Ligands (growth factors,
, SULF2, Ligands (growth factors,  ; chemokines,
; chemokines,  ;
;  morphogens).
morphogens).References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

