Evaluation of the Temporal Muscle Thickness as an Independent Prognostic Biomarker in Patients with Primary Central Nervous System Lymphoma
- PMID: 33540564
- PMCID: PMC7867149
- DOI: 10.3390/cancers13030566
Evaluation of the Temporal Muscle Thickness as an Independent Prognostic Biomarker in Patients with Primary Central Nervous System Lymphoma
Abstract
In this study, we assessed the prognostic relevance of temporal muscle thickness (TMT), likely reflecting patient's frailty, in patients with primary central nervous system lymphoma (PCNSL). In 128 newly diagnosed PCNSL patients TMT was analyzed on cranial magnetic resonance images. Predefined sex-specific TMT cutoff values were used to categorize the patient cohort. Survival analyses, using a log-rank test as well as Cox models adjusted for further prognostic parameters, were performed. The risk of death was significantly increased for PCNSL patients with reduced muscle thickness (hazard ratio of 3.189, 95% CI: 2-097-4.848, p < 0.001). Importantly, the results confirmed that TMT could be used as an independent prognostic marker upon multivariate Cox modeling (hazard ratio of 2.504, 95% CI: 1.608-3.911, p < 0.001) adjusting for sex, age at time of diagnosis, deep brain involvement of the PCNSL lesions, Eastern Cooperative Oncology Group (ECOG) performance status, and methotrexate-based chemotherapy. A TMT value below the sex-related cutoff value at the time of diagnosis is an independent adverse marker in patients with PCNSL. Thus, our results suggest the systematic inclusion of TMT in further translational and clinical studies designed to help validate its role as a prognostic biomarker.
Keywords: overall survival; primary central nervous system lymphoma; prognostic parameter; sarcopenia; temporal muscle thickness.
Conflict of interest statement
M.P. has received honoraria for lectures, consultation, or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, and Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by M.P. with payments made to his institution: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, and AbbVie. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
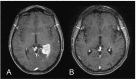
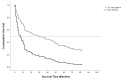

References
-
- Wöhrer A., Waldhör T., Heinzl H., Hackl M., Feichtinger J., Gruber-Mösenbacher U., Kiefer A., Maier H., Motz R., Reiner-Concin A., et al. The Austrian Brain Tumour Registry: A cooperative way to establish a population-based brain tumour registry. J. Neurooncol. 2009;95:401–411. doi: 10.1007/s11060-009-9938-9. - DOI - PubMed
-
- Ostrom Q.T., Gittleman H., Liao P., Vecchiobe-Koval T., Wolinsky Y., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(Suppl. 5):v1–v88. doi: 10.1093/neuonc/nox158. - DOI - PMC - PubMed
-
- Rubenstein J.L., Hsi E.D., Johnson J.L., Jung S.-H., Nakashima M.O., Grant B., Cheson B.D., Kaplan L.D. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202) J. Clin. Oncol. 2013;31:3061–3068. doi: 10.1200/JCO.2012.46.9957. - DOI - PMC - PubMed
-
- Omuro A., Correa D.D., DeAngelis L.M., Moskowitz C.H., Matasar M.J., Kaley T.J., Gavrilovic I.T., Nolan C., Pentsova E., Grommes C.C., et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403–1410. doi: 10.1182/blood-2014-10-604561. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

