Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management
- PMID: 33540569
- PMCID: PMC7867179
- DOI: 10.3390/jcm10030536
Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management
Abstract
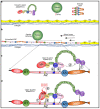
Thrombotic thrombocytopenic purpura (TTP) is a rare thrombotic microangiopathy characterized by microangiopathic hemolytic anemia, severe thrombocytopenia, and ischemic end organ injury due to microvascular platelet-rich thrombi. TTP results from a severe deficiency of the specific von Willebrand factor (VWF)-cleaving protease, ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13). ADAMTS13 deficiency is most commonly acquired due to anti-ADAMTS13 autoantibodies. It can also be inherited in the congenital form as a result of biallelic mutations in the ADAMTS13 gene. In adults, the condition is most often immune-mediated (iTTP) whereas congenital TTP (cTTP) is often detected in childhood or during pregnancy. iTTP occurs more often in women and is potentially lethal without prompt recognition and treatment. Front-line therapy includes daily plasma exchange with fresh frozen plasma replacement and immunosuppression with corticosteroids. Immunosuppression targeting ADAMTS13 autoantibodies with the humanized anti-CD20 monoclonal antibody rituximab is frequently added to the initial therapy. If available, anti-VWF therapy with caplacizumab is also added to the front-line setting. While it is hypothesized that refractory TTP will be less common in the era of caplacizumab, in relapsed or refractory cases cyclosporine A, N-acetylcysteine, bortezomib, cyclophosphamide, vincristine, or splenectomy can be considered. Novel agents, such as recombinant ADAMTS13, are also currently under investigation and show promise for the treatment of TTP. Long-term follow-up after the acute episode is critical to monitor for relapse and to diagnose and manage chronic sequelae of this disease.
Keywords: ADAMTS13; TTP; caplacizumab; diagnosis; follow-up; review; thrombotic thrombocytopenic purpura; treatment.
Conflict of interest statement
S.S. has no conflict of interest; B.L. is chairman of the Data Safety Monitoring Committees for studies on recombinant ADAMTS13 in congenital and acquired TTP (now run by Takeda); he is on the Advisory Board of Sanofi for the development of caplacizumab for acquired TTP; he received congress travel support and/or lecture fees from Ablynx, Alexion, Siemens, Bayer, Roche, and Sanofi; S.R.C. has received research funding and consulting/speaking fees from Sanofi-Genzyme and Takeda.
Figures

References
-
- Moschcowitz E. Hyaline Thrombosis of the Terminal Arterioles and Capillaries: A Hitherto Undescribed Disease. Proc. N. Y. Pathol. Soc. 1924;24:21–24.
-
- Amorosi E.L., Ultmann J.E. Thrombotic Thrombocytopenic Pupura: Report of 16 cases and Review of the Literature. Medicine. 1966;45:139–159. doi: 10.1097/00005792-196603000-00003. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

