CLytA-DAAO Chimeric Enzyme Bound to Magnetic Nanoparticles. A New Therapeutical Approach for Cancer Patients?
- PMID: 33540681
- PMCID: PMC7867295
- DOI: 10.3390/ijms22031477
CLytA-DAAO Chimeric Enzyme Bound to Magnetic Nanoparticles. A New Therapeutical Approach for Cancer Patients?
Abstract
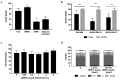
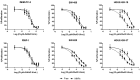
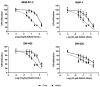
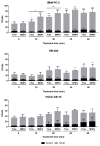
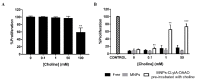
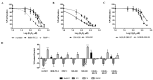
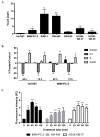
D-amino acid oxidase (DAAO) is an enzyme that catalyzes the oxidation of D-amino acids generating H2O2. The enzymatic chimera formed by DAAO bound to the choline-binding domain of N-acetylmuramoyl-L-alanine amidase (CLytA) induces cytotoxicity in several pancreatic and colorectal carcinoma and glioblastoma cell models. In the current work, we determined whether the effect of CLytA-DAAO immobilized in magnetic nanoparticles, gold nanoparticles, and alginate capsules offered some advantages as compared to the free CLytA-DAAO. Results indicate that the immobilization of CLytA-DAAO in magnetic nanoparticles increases the stability of the enzyme, extending its time of action. Besides, we compared the effect induced by CLytA-DAAO with the direct addition of hydrogen peroxide, demonstrating that the progressive generation of reactive oxygen species by CLytA-DAAO is more effective in inducing cytotoxicity than the direct addition of H2O2. Furthermore, a pilot study has been initiated in biopsies obtained from pancreatic and colorectal carcinoma and glioblastoma patients to evaluate the expression of the main genes involved in resistance to CLytA-DAAO cytotoxicity. Based on our findings, we propose that CLytA-DAAO immobilized in magnetic nanoparticles could be effective in a high percentage of patients and, therefore, be used as an anti-cancer therapy for pancreatic and colorectal carcinoma and glioblastoma.
Keywords: alginate capsules; cytotoxicity; enzymatic therapy; gold nanoparticle; hydrogen peroxide; magnetic nanoparticle; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
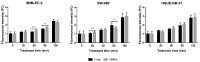
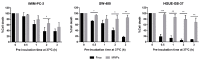

References
-
- Ali Khan A., Alzohairy M.A. Recent Advances and Applications of Immobilized Enzyme Technologies: A Review. Res. J. Biol. Sci. 2010;5:565–575. doi: 10.3923/rjbsci.2010.565.575. - DOI
MeSH terms
Substances
Grants and funding
- UGP-19-063/Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana
- PRECIPITA crowdfunding/Fundación Española para la Ciencia y la Tecnología
- Donation/Association of women affected by breast cancer in Elche and the region (AMACMEC)
- CP19/00095/Instituto de Salud Carlos III
- BIO2013-47684-R, BIO2016-79323-R/Ministerio de Economía, Industria y Competitividad, Gobierno de España
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

