Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus lopadusanus
- PMID: 33540688
- PMCID: PMC7913021
- DOI: 10.3390/antibiotics10020148
Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus lopadusanus
Abstract
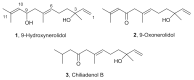
Chiliadenus lopadusanus Brullo is an Asteraceae plant species endemic to Lampedusa island, the largest island of the Pelage archipelago, Italy. The organic extract of its whole aerial parts, showing antibiotic activity against Staphylococcus aureus and Acinetobacter baumannii, wasfractionated employing bioguided purification procedures affording three main farnesane-type sesquiterpenoids. They were identified by spectroscopic methods (NMR and ESIMS data) as the (E)-3,7,11-trimethyldodeca-1,6,10-triene-3,9-diol, (E)-10-hydroxy-2,6,10-trimethyldodeca-2,6,11- trien-4-one and (E)-10-hydroxy-2,6,10-trimethyl-dodeca-6,11-dien-4-one, commonly named 9-hydroxynerolidol, 9-oxonerolidol, and chiliadenol B, respectively. These three sesquiterpenes, isolated for the first time from C. lopadusanus, were tested on methicillin-resistant S. aureus and A. baumannii showing antibacterial and antibiofilm activities. This plant could be used as a source to isolate secondary metabolites as potential new antibiotics.
Keywords: Chiliadenus lopadusanus; antibacterial activity; antibiofilm activity; sesquiterpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization (WHO) Antimicrobial Resistance No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. [(accessed on 8 April 2020)]; Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-gr....
LinkOut - more resources
Full Text Sources
Other Literature Sources

