Sulfonate-Conjugated Polyelectrolytes as Anode Interfacial Layers in Inverted Organic Solar Cells
- PMID: 33540730
- PMCID: PMC7867262
- DOI: 10.3390/molecules26030763
Sulfonate-Conjugated Polyelectrolytes as Anode Interfacial Layers in Inverted Organic Solar Cells
Abstract
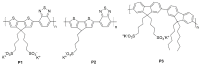
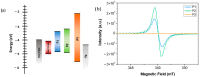
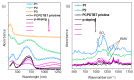
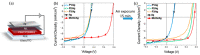
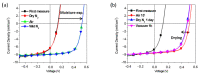
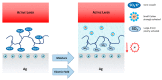
Conjugated polymers with ionic pendant groups (CPEs) are receiving increasing attention as solution-processed interfacial materials for organic solar cells (OSCs). Various anionic CPEs have been successfully used, on top of ITO (Indium Tin Oxide) electrodes, as solution-processed anode interlayers (AILs) for conventional devices with direct geometry. However, the development of CPE AILs for OSC devices with inverted geometry is an important topic that still needs to be addressed. Here, we have designed three anionic CPEs bearing alkyl-potassium-sulfonate side chains. Their functional behavior as anode interlayers has been investigated in P3HT:PC61BM (poly(3-hexylthiophene): [6,6]-phenyl C61 butyric acid methyl ester) devices with an inverted geometry, using a hole collecting silver electrode evaporated on top. Our results reveal that to obtain effective anode modification, the CPEs' conjugated backbone has to be tailored to grant self-doping and to have a good energy-level match with the photoactive layer. Furthermore, the sulfonate moieties not only ensure the solubility in polar orthogonal solvents, induce self-doping via a right choice of the conjugated backbone, but also play a role in the gaining of hole selectivity of the top silver electrode.
Keywords: anode interfacial layers; conjugated polyelectrolytes; inverted organic solar cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Steim R., Kogler F.R., Brabec C.J. Interface materials for organic solar cells. J. Mater. Chem. 2010;20:2499–2512. doi: 10.1039/b921624c. - DOI
-
- Yip H.-L., Jen A.K.-Y. Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Energy Environ. Sci. 2012;5:5994–6011. doi: 10.1039/c2ee02806a. - DOI
-
- Corzo D., Bihar E., Alexandre E.B., Rosas-Villalva D., Baran D. Ink Engineering of Transport Layers for 9.5% Efficient All-Printed Semitransparent Nonfullerene Solar Cells. Adv. Funct. Mater. 2020;2005763 doi: 10.1002/adfm.202005763. - DOI
-
- Chueh C.-C., Li C.-Z., Jen A.K.-Y. Recent progress and perspective in solution-processed Interfacial materials for efficient and stable polymer and organometal perovskite solar cells. Energy Environ. Sci. 2015;8:1160–1189. doi: 10.1039/C4EE03824J. - DOI
-
- Jiang Y., Peng H., Mai R., Meng Y., Rong Q., Cabanetos C., Nian L., Roncali J., Zhou G., Liu J., et al. Alcohol-soluble anode modifier for highly efficient inverted solar cells with oligo-oxyethylene chains. Org. Electron. 2019;68:200–204. doi: 10.1016/j.orgel.2019.01.012. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

