Local anesthetics impair the growth and self-renewal of glioblastoma stem cells by inhibiting ZDHHC15-mediated GP130 palmitoylation
- PMID: 33541421
- PMCID: PMC7863430
- DOI: 10.1186/s13287-021-02175-2
Local anesthetics impair the growth and self-renewal of glioblastoma stem cells by inhibiting ZDHHC15-mediated GP130 palmitoylation
Abstract
Background: A large number of preclinical studies have shown that local anesthetics have a direct inhibitory effect on tumor biological activities, including cell survival, proliferation, migration, and invasion. There are few studies on the role of local anesthetics in cancer stem cells. This study aimed to determine the possible role of local anesthetics in glioblastoma stem cell (GSC) self-renewal and the underlying molecular mechanisms.
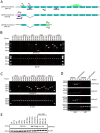
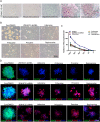
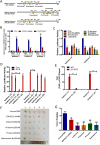
Methods: The effects of local anesthetics in GSCs were investigated through in vitro and in vivo assays (i.e., Cell Counting Kit 8, spheroidal formation assay, double immunofluorescence, western blot, and xenograft model). The acyl-biotin exchange method (ABE) assay was identified proteins that are S-acylated by zinc finger Asp-His-His-Cys-type palmitoyltransferase 15 (ZDHHC15). Western blot, co-immunoprecipitation, and liquid chromatograph mass spectrometer-mass spectrometry assays were used to explore the mechanisms of ZDHHC15 in effects of local anesthetics in GSCs.
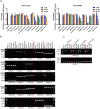
Results: In this study, we identified a novel mechanism through which local anesthetics can damage the malignant phenotype of glioma. We found that local anesthetics prilocaine, lidocaine, procaine, and ropivacaine can impair the survival and self-renewal of GSCs, especially the classic glioblastoma subtype. These findings suggest that local anesthetics may weaken ZDHHC15 transcripts and decrease GP130 palmitoylation levels and membrane localization, thus inhibiting the activation of IL-6/STAT3 signaling.
Conclusions: In conclusion, our work emphasizes that ZDHHC15 is a candidate therapeutic target, and local anesthetics are potential therapeutic options for glioblastoma.
Keywords: GP130; Glioblastoma stem cells; Local anesthetics; STAT3; Self-renewal; ZDHHC15.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Tetraspanin CD9 stabilizes gp130 by preventing its ubiquitin-dependent lysosomal degradation to promote STAT3 activation in glioma stem cells.Cell Death Differ. 2017 Jan;24(1):167-180. doi: 10.1038/cdd.2016.110. Epub 2016 Oct 14. Cell Death Differ. 2017. PMID: 27740621 Free PMC article.
-
S-palmitoylation of c-MET by CK2α-mediated zDHHC15 phosphorylation drives glioblastoma stem cell tumorigenicity.Neuro Oncol. 2025 Apr 10:noaf098. doi: 10.1093/neuonc/noaf098. Online ahead of print. Neuro Oncol. 2025. PMID: 40207776
-
Oct4A palmitoylation modulates tumorigenicity and stemness in human glioblastoma cells.Neuro Oncol. 2023 Jan 5;25(1):82-96. doi: 10.1093/neuonc/noac157. Neuro Oncol. 2023. PMID: 35727735 Free PMC article.
-
Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells.Stem Cell Res Ther. 2017 Apr 17;8(1):76. doi: 10.1186/s13287-017-0518-1. Stem Cell Res Ther. 2017. PMID: 28412969 Free PMC article.
-
Ibrutinib inactivates BMX-STAT3 in glioma stem cells to impair malignant growth and radioresistance.Sci Transl Med. 2018 May 30;10(443):eaah6816. doi: 10.1126/scitranslmed.aah6816. Sci Transl Med. 2018. PMID: 29848664 Free PMC article.
Cited by
-
Protein palmitoylation in cancer: molecular functions and therapeutic potential.Mol Oncol. 2023 Jan;17(1):3-26. doi: 10.1002/1878-0261.13308. Epub 2022 Sep 10. Mol Oncol. 2023. PMID: 36018061 Free PMC article. Review.
-
Research progress on S-palmitoylation modification mediated by the ZDHHC family in glioblastoma.Front Cell Dev Biol. 2024 Nov 5;12:1413708. doi: 10.3389/fcell.2024.1413708. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39563863 Free PMC article. Review.
-
2-Bromopalmitate inhibits malignant behaviors of HPSCC cells by hindering the membrane location of Ras protein.Exp Biol Med (Maywood). 2023 Dec;248(23):2393-2407. doi: 10.1177/15353702231220671. Epub 2023 Dec 30. Exp Biol Med (Maywood). 2023. PMID: 38159074 Free PMC article.
-
LINC02308 promotes the progression of glioma through activating mTOR/AKT-signaling pathway by targeting miR-30e-3p/TM4SF1 axis.Cell Biol Toxicol. 2022 Apr;38(2):223-236. doi: 10.1007/s10565-021-09604-1. Epub 2021 May 4. Cell Biol Toxicol. 2022. PMID: 33945031
-
The effect of lidocaine infusion in oncologic surgery: A bibliometric analysis based on CiteSpace.Medicine (Baltimore). 2024 Dec 20;103(51):e40980. doi: 10.1097/MD.0000000000040980. Medicine (Baltimore). 2024. PMID: 39705410 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

