Engineering advanced logic and distributed computing in human CAR immune cells
- PMID: 33542232
- PMCID: PMC7862674
- DOI: 10.1038/s41467-021-21078-7
Engineering advanced logic and distributed computing in human CAR immune cells
Abstract
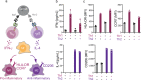
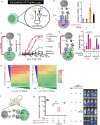
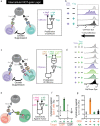
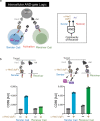
The immune system is a sophisticated network of different cell types performing complex biocomputation at single-cell and consortium levels. The ability to reprogram such an interconnected multicellular system holds enormous promise in treating various diseases, as exemplified by the use of chimeric antigen receptor (CAR) T cells as cancer therapy. However, most CAR designs lack computation features and cannot reprogram multiple immune cell types in a coordinated manner. Here, leveraging our split, universal, and programmable (SUPRA) CAR system, we develop an inhibitory feature, achieving a three-input logic, and demonstrate that this programmable system is functional in diverse adaptive and innate immune cells. We also create an inducible multi-cellular NIMPLY circuit, kill switch, and a synthetic intercellular communication channel. Our work highlights that a simple split CAR design can generate diverse and complex phenotypes and provide a foundation for engineering an immune cell consortium with user-defined functionalities.
Conflict of interest statement
The authors declare the following competing interests: Boston University has filed a patent application, WO2017091546A1, (Methods and compositions relating to chimeric antigen receptors) with J.H.C, and W.W.W. as the named inventor based on this work. W.W.W. is a co-founder and shareholder of Senti Biosciences, and received research support from Senti Biosciences. J.J.C. is a co-founder and shareholder of Senti Biosciences. A.O. is a current employee of Hitachi, Ltd. J.H.C. is a current employee of Spark Therapeutics. K.S. is current employee of KSQ Therapeutics. All other authors declare no competing interests.
Figures



References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

