Chondrogenesis of human amniotic fluid stem cells in Chitosan-Xanthan scaffold for cartilage tissue engineering
- PMID: 33542256
- PMCID: PMC7862244
- DOI: 10.1038/s41598-021-82341-x
Chondrogenesis of human amniotic fluid stem cells in Chitosan-Xanthan scaffold for cartilage tissue engineering
Abstract
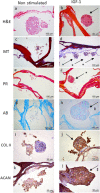
Articular chondral lesions, caused either by trauma or chronic cartilage diseases such as osteoarthritis, present very low ability to self-regenerate. Thus, their current management is basically symptomatic, progressing very often to invasive procedures or even arthroplasties. The use of amniotic fluid stem cells (AFSCs), due to their multipotentiality and plasticity, associated with scaffolds, is a promising alternative for the reconstruction of articular cartilage. Therefore, this study aimed to investigate the chondrogenic potential of AFSCs in a micromass system (high-density cell culture) under insulin-like growth factor 1 (IGF-1) stimuli, as well as to look at their potential to differentiate directly when cultured in a porous chitosan-xanthan (CX) scaffold. The experiments were performed with a CD117 positive cell population, with expression of markers (CD117, SSEA-4, Oct-4 and NANOG), selected from AFSCs, after immunomagnetic separation. The cells were cultured in both a micromass system and directly in the scaffold, in the presence of IGF-1. Differentiation to chondrocytes was confirmed by histology and by using immunohistochemistry. The construct cell-scaffold was also analyzed by scanning electron microscopy (SEM). The results demonstrated the chondrogenic potential of AFSCs cultivated directly in CX scaffolds and also in the micromass system. Such findings support and stimulate future studies using these constructs in osteoarthritic animal models.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

