Probiotics as a treatment for prenatal maternal anxiety and depression: a double-blind randomized pilot trial
- PMID: 33542275
- PMCID: PMC7862351
- DOI: 10.1038/s41598-021-81204-9
Probiotics as a treatment for prenatal maternal anxiety and depression: a double-blind randomized pilot trial
Abstract
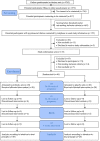
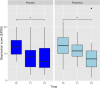
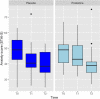
Probiotic use may be an efficacious treatment option to effectively manage symptoms of prenatal maternal anxiety and depression. Our primary aim was to test feasibility and acceptability for a probiotic randomized controlled trial (RCT) in pregnant women with pre-existing symptoms. This double-blind pilot RCT included 40 pregnant women with low-risk pregnancies and elevated depressive symptoms and/or anxiety. Once daily, participants orally consumed a probiotic (Ecologic Barrier) or a placebo, from 26 to 30 weeks gestation until delivery. A priori key progression criteria for primary outcomes were determined to decide whether or not a full RCT was feasible and acceptable. Secondary outcomes included depressive symptoms, anxiety, stress, and maternal bonding to offspring. In 19 months, 1573 women were screened; following screening, 155 women (10%) were invited for participation, of whom 135 (87%) received study information, and 40 women (30%) were included. Four out of six a priori determined criteria for success on feasibility and acceptability were met. After 8 weeks of intervention, there was no significant difference between the probiotic and placebo groups for secondary outcomes. The pilot trial was feasible and acceptable, but hampered by recruitment method and study design. Secondary endpoints did not reveal differences between the groups for improving maternal mood.
Conflict of interest statement
Eric Claassen is a consultant for several commercial parties in the field of probiotics; none of his advising practices are in direct conflict with the content of this pilot trial. Isolde Besseling-Van der Vaart is employed at Winclove Probiotics B.V. fulfilling the function of Manager Research Partnerships. The other authors have no conflict of interest.
Figures

Similar articles
-
Probiotics in pregnancy: protocol of a double-blind randomized controlled pilot trial for pregnant women with depression and anxiety (PIP pilot trial).Trials. 2019 Jul 17;20(1):440. doi: 10.1186/s13063-019-3389-1. Trials. 2019. PMID: 31315657 Free PMC article.
-
Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial.EBioMedicine. 2017 Oct;24:159-165. doi: 10.1016/j.ebiom.2017.09.013. Epub 2017 Sep 14. EBioMedicine. 2017. PMID: 28943228 Free PMC article. Clinical Trial.
-
Effects of probiotics (Vivomixx®) in obese pregnant women and their newborn: study protocol for a randomized controlled trial.Trials. 2016 Oct 11;17(1):491. doi: 10.1186/s13063-016-1617-5. Trials. 2016. PMID: 27724923 Free PMC article. Clinical Trial.
-
Adjuvant therapy with antidepressants for the management of inflammatory bowel disease.Cochrane Database Syst Rev. 2019 Apr 12;4(4):CD012680. doi: 10.1002/14651858.CD012680.pub2. Cochrane Database Syst Rev. 2019. PMID: 30977111 Free PMC article.
-
Universal prevention of distress aimed at pregnant women: a systematic review and meta-analysis of psychological interventions.BMC Pregnancy Childbirth. 2021 Apr 1;21(1):276. doi: 10.1186/s12884-021-03752-2. BMC Pregnancy Childbirth. 2021. PMID: 33794828 Free PMC article.
Cited by
-
Maternal mood, anxiety and mental health functioning after combined myo-inositol, probiotics, micronutrient supplementation from preconception: Findings from the NiPPeR RCT.Psychiatry Res. 2024 Apr;334:115813. doi: 10.1016/j.psychres.2024.115813. Epub 2024 Feb 23. Psychiatry Res. 2024. PMID: 38402742 Free PMC article. Clinical Trial.
-
A Longitudinal Observational Study of a Powder-Based Supplement for a Future Synbiotic Trial in Breastfed Children From South Africa.Sage Open Pediatr. 2025 Jul 11;12:2333794X241309003. doi: 10.1177/2333794X241309003. eCollection 2025 Jan-Dec. Sage Open Pediatr. 2025. PMID: 40655430 Free PMC article.
-
The Maternal Microbiome as a Map to Understanding the Impact of Prenatal Stress on Offspring Psychiatric Health.Biol Psychiatry. 2024 Feb 15;95(4):300-309. doi: 10.1016/j.biopsych.2023.11.014. Epub 2023 Nov 30. Biol Psychiatry. 2024. PMID: 38042328 Free PMC article. Review.
-
An Updated Narrative Mini-Review on the Microbiota Changes in Antenatal and Post-Partum Depression.Diagnostics (Basel). 2022 Jun 28;12(7):1576. doi: 10.3390/diagnostics12071576. Diagnostics (Basel). 2022. PMID: 35885482 Free PMC article. Review.
-
The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies.Int J Mol Sci. 2022 Apr 19;23(9):4494. doi: 10.3390/ijms23094494. Int J Mol Sci. 2022. PMID: 35562885 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

