Gametes deficient for Pot1 telomere binding proteins alter levels of telomeric foci for multiple generations
- PMID: 33542458
- PMCID: PMC7862594
- DOI: 10.1038/s42003-020-01624-7
Gametes deficient for Pot1 telomere binding proteins alter levels of telomeric foci for multiple generations
Abstract
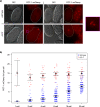
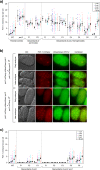
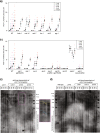
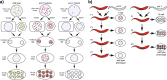
Deficiency for telomerase results in transgenerational shortening of telomeres. However, telomeres have no known role in transgenerational epigenetic inheritance. C. elegans Protection Of Telomeres 1 (Pot1) proteins form foci at the telomeres of germ cells that disappear at fertilization and gradually accumulate during development. We find that gametes from mutants deficient for Pot1 proteins alter levels of telomeric foci for multiple generations. Gametes from pot-2 mutants give rise to progeny with abundant POT-1::mCherry and mNeonGreen::POT-2 foci throughout development, which persists for six generations. In contrast, gametes from pot-1 mutants or pot-1; pot-2 double mutants induce diminished Pot1 foci for several generations. Deficiency for MET-2, SET-25, or SET-32 methyltransferases, which promote heterochromatin formation, results in gametes that induce diminished Pot1 foci for several generations. We propose that C. elegans POT-1 may interact with H3K9 methyltransferases during pot-2 mutant gametogenesis to induce a persistent form of transgenerational epigenetic inheritance that causes constitutively high levels of heterochromatic Pot1 foci.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Genetic and Epigenetic Inheritance at Telomeres.Epigenomes. 2022 Mar 16;6(1):9. doi: 10.3390/epigenomes6010009. Epigenomes. 2022. PMID: 35323213 Free PMC article. Review.
-
Caenorhabditis elegans POT-1 and POT-2 repress telomere maintenance pathways.G3 (Bethesda). 2013 Feb;3(2):305-13. doi: 10.1534/g3.112.004440. Epub 2013 Feb 1. G3 (Bethesda). 2013. PMID: 23390606 Free PMC article.
-
Telomeric double-strand DNA-binding proteins DTN-1 and DTN-2 ensure germline immortality in Caenorhabditis elegans.Elife. 2021 Jan 21;10:e64104. doi: 10.7554/eLife.64104. Elife. 2021. PMID: 33476260 Free PMC article.
-
The double-stranded DNA-binding proteins TEBP-1 and TEBP-2 form a telomeric complex with POT-1.Nat Commun. 2021 May 11;12(1):2668. doi: 10.1038/s41467-021-22861-2. Nat Commun. 2021. PMID: 33976151 Free PMC article.
-
POT1 mutations cause differential effects on telomere length leading to opposing disease phenotypes.J Cell Physiol. 2023 Jun;238(6):1237-1255. doi: 10.1002/jcp.31034. Epub 2023 May 14. J Cell Physiol. 2023. PMID: 37183325 Review.
Cited by
-
Caenorhabditis elegans for research on cancer hallmarks.Dis Model Mech. 2023 Jun 1;16(6):dmm050079. doi: 10.1242/dmm.050079. Epub 2023 Jun 6. Dis Model Mech. 2023. PMID: 37278614 Free PMC article.
-
Genetic and Epigenetic Inheritance at Telomeres.Epigenomes. 2022 Mar 16;6(1):9. doi: 10.3390/epigenomes6010009. Epigenomes. 2022. PMID: 35323213 Free PMC article. Review.
-
Low-Dose Radiation Can Cause Epigenetic Alterations Associated With Impairments in Both Male and Female Reproductive Cells.Front Genet. 2021 Aug 2;12:710143. doi: 10.3389/fgene.2021.710143. eCollection 2021. Front Genet. 2021. PMID: 34408775 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

