Activity-dependent regulome of human GABAergic neurons reveals new patterns of gene regulation and neurological disease heritability
- PMID: 33542524
- PMCID: PMC7933108
- DOI: 10.1038/s41593-020-00786-1
Activity-dependent regulome of human GABAergic neurons reveals new patterns of gene regulation and neurological disease heritability
Abstract
Neuronal activity-dependent gene expression is essential for brain development. Although transcriptional and epigenetic effects of neuronal activity have been explored in mice, such an investigation is lacking in humans. Because alterations in GABAergic neuronal circuits are implicated in neurological disorders, we conducted a comprehensive activity-dependent transcriptional and epigenetic profiling of human induced pluripotent stem cell-derived GABAergic neurons similar to those of the early developing striatum. We identified genes whose expression is inducible after membrane depolarization, some of which have specifically evolved in primates and/or are associated with neurological diseases, including schizophrenia and autism spectrum disorder (ASD). We define the genome-wide profile of human neuronal activity-dependent enhancers, promoters and the transcription factors CREB and CRTC1. We found significant heritability enrichment for ASD in the inducible promoters. Our results suggest that sequence variation within activity-inducible promoters of developing human forebrain GABAergic neurons contributes to ASD risk.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
Figures





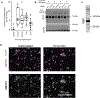



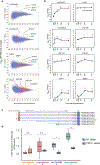



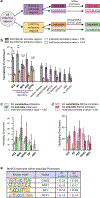
References
MAIN TEXT REFERENCES
-
- Hensch TK Critical period regulation. Annual review of neuroscience 27, 549–79 (2004). - PubMed
METHODS ONLY REFERENCES
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

