Salbutamol for transient tachypnea of the newborn
- PMID: 33543473
- PMCID: PMC8094231
- DOI: 10.1002/14651858.CD011878.pub3
Salbutamol for transient tachypnea of the newborn
Abstract
Background: Transient tachypnea of the newborn is characterized by tachypnea and signs of respiratory distress. Transient tachypnea typically appears within the first two hours of life in term and late preterm newborns. Although transient tachypnea of the newborn is usually a self-limited condition, it is associated with wheezing syndromes in late childhood. The rationale for the use of salbutamol (albuterol) for transient tachypnea of the newborn is based on studies showing that β-agonists can accelerate the rate of alveolar fluid clearance. This review was originally published in 2016 and updated in 2020.
Objectives: To assess whether salbutamol compared to placebo, no treatment or any other drugs administered to treat transient tachypnea of the newborn, is effective and safe for infants born at 34 weeks' gestational age with this diagnosis.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2020, Issue 4) in the Cochrane Library; PubMed (1996 to April 2020), Embase (1980 to April 2020); and CINAHL (1982 to April 2020). We applied no language restrictions. We searched the abstracts of the major congresses in the field (Perinatal Society of Australia New Zealand and Pediatric Academic Societies) from 2000 to 2020 and clinical trial registries.
Selection criteria: Randomized controlled trials, quasi-randomized controlled trials and cluster trials comparing salbutamol versus placebo or no treatment or any other drugs administered to infants born at 34 weeks' gestational age or more and less than three days of age with transient tachypnea of the newborn.
Data collection and analysis: We used standard Cochrane methodology for data collection and analysis. The primary outcomes considered in this review were duration of oxygen therapy, need for continuous positive airway pressure and need for mechanical ventilation. We used the GRADE approach to assess the certainty of evidence.
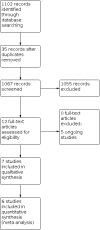
Main results: Seven trials, which included 498 infants, met the inclusion criteria. All trials compared a nebulized dose of salbutamol with normal saline. Four studies used one single dose of salbutamol; in two studies, three to four doses were provided; in one study, additional doses were administered if needed. The certainty of the evidence was low for duration of hospital stay and very low for the other outcomes. Among the primary outcomes of this review, four trials (338 infants) reported the duration of oxygen therapy, (mean difference (MD) -19.24 hours, 95% confidence interval (CI) -23.76 to -14.72); one trial (46 infants) reported the need for continuous positive airway pressure (risk ratio (RR) 0.73, 95% CI 0.38 to 1.39; risk difference (RD) -0.15, 95% CI -0.45 to 0.16), and three trials (254 infants) reported the need for mechanical ventilation (RR 0.60, 95% CI 0.13 to 2.86; RD -0.01, 95% CI -0.05 to 0.03). Both duration of hospital stay (4 trials; 338 infants) and duration of respiratory support (2 trials, 228 infants) were shorter in the salbutamol group (MD -1.48, 95% CI -1.8 to -1.16; MD -9.24, 95% CI -14.24 to -4.23, respectively). One trial (80 infants) reported duration of mechanical ventilation and pneumothorax but data could not be extracted due to the reporting of these outcomes (type of units of effect measure and unclear number of events, respectively). Five trials are ongoing.
Authors' conclusions: There was limited evidence to establish the benefits and harms of salbutamol in the management of transient tachypnea of the newborn. We are uncertain whether salbutamol administration reduces the duration of oxygen therapy, duration of tachypnea, need for continuous positive airway pressure and for mechanical ventilation. Salbutamol may slightly reduce hospital stay. Five trials are ongoing. Given the limited and low certainty of the evidence available, we could not determine whether salbutamol was safe or effective for the treatment of transient tachypnea of the newborn.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
LM has no interest to declare.
MB has received research funding from ALF grant (non‐profit ‐ Lund University) and Crafoord Foundation (non‐profit) for research projects not related to Cochrane.
MM has no interest to declare.
MGC has no interest to declare.
Figures












Update of
-
Salbutamol for transient tachypnea of the newborn.Cochrane Database Syst Rev. 2016 May 23;(5):CD011878. doi: 10.1002/14651858.CD011878.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Feb 5;2:CD011878. doi: 10.1002/14651858.CD011878.pub3. PMID: 27210618 Updated.
References
References to studies included in this review
Armangil 2011 {published data only}
Babaei 2019 {published data only}
-
- Babaei H, Dabiri S, Pirkashani LM, Mohsenpour H. Effects of salbutamol on the treatment of transient tachypnea of the newborn. Iranian Journal of Neonatology 2019;10(1):42-9. [DOI: 10.22038/IJN.2018.31294.1430] - DOI
Kim 2014 {published data only}
Malakian 2018 {published data only}
Mohammadzadeh 2017 {published data only}
-
- Mohammadzadeh I, Akbarian-Rad Z, Heidari F, Zahedpasha Y, Haghshenas-Mojaveri M. The effect of inhaled salbutamol in transient of tachypnea of the newborn: a randomized clinical trial. Iranian Journal of Pediatrics 2017;27(5):e9633. [DOI: 10.5812/ijp.9633] - DOI
Monzoy‐Ventre 2015 {published data only}
-
- Monzoy-Ventre MA, Rosas-Sumano AB, Hernández-Enríquez NP, Galicia-Flores L. Inhaled salbutamol in newborn infants with transient tachypnea [Salbutamol inhalado en los niños recién nacidos con taquipnea transitoria]. Revista Mexicana de Pediatria 2015;82(1):5-9.
Mussavi 2017 {published data only}
-
- Mussavi M, Asadollahi K, Kayvan M, Sadeghvand S. Effects of nebulized albuterol in transient tachypnea of the newborn a clinical trial. Iranian Journal of Pediatrics 2017;27(3):e8211. [DOI: 10.5812/ijp.8211] - DOI
References to ongoing studies
IRCT2014062518232N1 {published data only}
-
- IRCT2014062518232N1. The effect of salbutamol in treatment of transient tachypnea of newborn. irct.ir/searchresult.php?id=18232&number=1 (first received 7 August 2014).
IRCT2016010225811N1 {published data only}
-
- IRCT2016010225811N1. Investigation of the effects of nebulized ventolin in transient tachypnea of newborn [Investigation of the effects of nebulized ventolin and saline 0.%9 in transient respiratory distress of newborn]. irct.ir/trial/21548 (first received 18 March 2016).
IRCT201711139014N201 {published data only}
-
- IRCT201711139014N201. Effect of inhaler salbutamol versus placebo on treatment of neonatal transient tachypnea: a double blind randomized clinical trial [Effect of inhaler salbutamol versus placebo on treatment of neonatal transient tachypnea]. irct.ir/trial/9639 (first received 15 November 2017).
IRCT20190503043457N1 {published data only}
-
- IRCT20190503043457N1. Comparison of inhaled salbutamol with placebo (water for inj.) on recovery process of newborns' transient tachypnea [Comparison of inhaled salbutamol with placebo (water for inj.) as an add-therapies on the duration of stay of newborns and the recovery process of tachypnea and 02 dependence in transient tachypnea of newborns in NICU in Qom hospitals: a clinical trial study]. en.irct.ir/trial/39273 (first received 28 May 2019).
NCT03208894 {published data only}
-
- NCT03208894. Role of salbutamol and furosemide in TTN [Role of salbutamol and furosemide in transient tachypnea of newborn]. clinicaltrials.gov/ct2/show/NCT03208894 (first received 6 July 2017).
Additional references
Avery 1966
Barker 2002
Clark 2005
-
- Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. Journal of Perinatology 2005;25(4):251-7. [DOI: 10.1038/sj.jp.7211242] [PMID: ] - PubMed
Cochrane EPOC Group 2013
-
- Effective Practice and Organisation of Care (EPOC). Data extraction and management. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed 15 May 2016).
Davies 2004
Di Marco 2012
Faxelius 1983
Foster 2015
-
- Foster JP, Buckmaster A, Sinclair L, Lees S, Guaran R. Nasal continuous positive airway pressure (nCPAP) for term neonates with respiratory distress. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD011962. [DOI: 10.1002/14651858.CD011962] - DOI
Frank 2000
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 12 January 2021. Available at gradepro.org.
Guglani 2008
Gupta 2015
Hansen 2008
-
- Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ 2008;336(7635):85-7. [DOI: 10.1136/bmj.39405.539282.BE] [PMID: ] - DOI - PMC - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editor(s)). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. - PMC - PubMed
Karabayir 2010
Kassab 2015
Kumar 1996
Licker 2008
Liem 2007
Ma 2010
-
- Ma XL, Xu XF, Chen C, Yan CY, Liu YM, Liu L, et al, National Collaborative Study Group for Neonatal Respiratory Distress in Late Preterm or Term Infants. Epidemiology of respiratory distress and the illness severity in late preterm or term infants: a prospective multi-center study. Chinese Medical Journal 2010;123(20):2776-80. [PMID: ] - PubMed
Miller 1980
-
- Miller LK, Calenoff L, Boehm JJ, Riedy MJ. Respiratory distress in the newborn. JAMA 1980;243(11):1176-9. [PMID: ] - PubMed
Minakata 1998
Morrison 1995
Perkins 2006
Rawlings 1984
-
- Rawlings JS, Smith FR. Transient tachypnea of the newborn: an analysis of neonatal and obstetric risk factors. American Journal of Diseases of Children 1984;138:869-71. [PMID: ] - PubMed
RevMan 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Cochrane Collaboration, 2020.
Sakuma 1994
Sakuma 1996
Sartori 2002
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Silverman 1956
-
- Silverman WE, Anderson DH. Controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956;17:1-10. [PMID: ] - PubMed
Sterne 2017
-
- Sterne JAC, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbookfor Systematic Reviews of Interventions, Version 5.2.0 (updated June 2017), Cochrane. Available from training.cochrane.org/handbook 2017.
Stroustrup 2012
Tudehope 1979
References to other published versions of this review
Moresco 2015
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

