College of American Pathologists Tumor Regression Grading System for Long-Term Outcome in Patients with Locally Advanced Rectal Cancer
- PMID: 33543577
- PMCID: PMC8100558
- DOI: 10.1002/onco.13707
College of American Pathologists Tumor Regression Grading System for Long-Term Outcome in Patients with Locally Advanced Rectal Cancer
Abstract
Background: The National Comprehensive Cancer Network's Rectal Cancer Guideline Panel recommends American Joint Committee of Cancer and College of American Pathologists (AJCC/CAP) tumor regression grading (TRG) system to evaluate pathologic response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer (LARC). Yet, the clinical significance of the AJCC/CAP TRG system has not been fully defined.
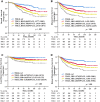
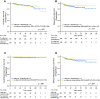
Materials and methods: This was a multicenter, retrospectively recruited, and prospectively maintained cohort study. Patients with LARC from one institution formed the discovery set, and cases from external independent institutions formed a validation set to verify the findings from discovery set. Overall survival (OS), disease-free survival (DFS), local recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS) were assessed by Kaplan-Meier analysis, log-rank test, and Cox regression model.
Results: The discovery set (940 cases) found, and the validation set (2,156 cases) further confirmed, that inferior AJCC/CAP TRG categories were closely /ccorrelated with unfavorable survival (OS, DFS, LRFS, and DMFS) and higher risk of disease progression (death, accumulative relapse, local recurrence, and distant metastasis) (all p < .05). Significantly, pairwise comparison revealed that any two of four TRG categories had the distinguished survival and risk of disease progression. After propensity score matching, AJCC/CAP TRG0 category (pathological complete response) patients treated with or without adjuvant chemotherapy displayed similar survival of OS, DFS, LRFS, and DMFS (all p > .05). For AJCC/CAP TRG1-3 cases, adjuvant chemotherapy treatment significantly improved 3-year OS (90.2% vs. 84.6%, p < .001). Multivariate analysis demonstrated the AJCC/CAP TRG system was an independent prognostic surrogate.
Conclusion: AJCC/CAP TRG system, an accurate prognostic surrogate, appears ideal for further strategizing adjuvant chemotherapy for LARC.
Implications for practice: The National Comprehensive Cancer Network recommends the American Joint Committee of Cancer and College of American Pathologists (AJCC/CAP) tumor regression grading (TRG) four-category system to evaluate the pathologic response to neoadjuvant treatment for patients with locally advanced rectal cancer; however, the clinical significance of the AJCC/CAP TRG system has not yet been clearly addressed. This study found, for the first time, that any two of four AJCC/CAP TRG categories had the distinguished long-term survival outcome. Importantly, adjuvant chemotherapy may improve the 3-year overall survival for AJCC/CAP TRG1-3 category patients but not for AJCC/CAP TRG0 category patients. Thus, AJCC/CAP TRG system, an accurate surrogate of long-term survival outcome, is useful in guiding adjuvant chemotherapy management for rectal cancer.
Keywords: Adjuvant chemotherapy; Locally advanced rectal cancer; Neoadjuvant treatments; Survival outcome; Tumor regression grade system.
© 2021 AlphaMed Press.
Conflict of interest statement
Figures

References
-
- Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. - PubMed
-
- Maas M, Nelemans PJ, Valentini V et al. Long‐term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 2010;11:835–844. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

