Physiology and theory of tracer washout techniques for the estimation of myocardial blood flow: flow estimation from tracer washout
- PMID: 335437
- PMCID: PMC3021479
- DOI: 10.1016/0033-0620(77)90019-6
Physiology and theory of tracer washout techniques for the estimation of myocardial blood flow: flow estimation from tracer washout
Abstract
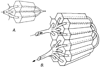
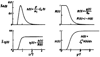
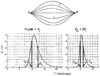
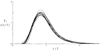
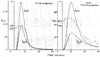
The time course of washout of tracer from the myocardium provides an estimate of the flow per unit volume when the blood-tissue exchange is flow-limited. Methods of testing for the flow-limitation and for the absence of influences of low permeability or diffusion on the washout include the uses of paired or multiple tracers and the examination for similarity of the shapes of the residue function or washout curves at varied coronary blood flows. A conceptual framework for these studies is provided by a clearance-flow diagram for the myocardium where capillaries are long compared to radial intercapillary distances. This anatomic-physiologic framework coupled with a probabilistic, general analytic approach and with various experimental approaches to tracer studies of mass transport through the heart provides a general basis for methods of estimating myocardial blood flow in the whole organ and in its component regions.
Figures














References
-
- Zierler KL. Equations for measuring blood flow by external monitoring of radioisotopes. Circ Res. 1965;16:309–321. - PubMed
-
- Chinard FP, Enns T, Nolan MF. Indicator–dilution studies with “diffusible” indicators. Circ Res. 1962;10:473, 490. - PubMed
-
- Renkin EM. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physio1. 1959;197:1205–1210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

