Multiomics-Identified Intervention to Restore Ethanol-Induced Dysregulated Proteostasis and Secondary Sarcopenia in Alcoholic Liver Disease
- PMID: 33543862
- PMCID: PMC8195260
- DOI: 10.33594/000000327
Multiomics-Identified Intervention to Restore Ethanol-Induced Dysregulated Proteostasis and Secondary Sarcopenia in Alcoholic Liver Disease
Abstract
Background/aims: Signaling and metabolic perturbations contribute to dysregulated skeletal muscle protein homeostasis and secondary sarcopenia in response to a number of cellular stressors including ethanol exposure. Using an innovative multiomics-based curating of unbiased data, we identified molecular and metabolic therapeutic targets and experimentally validated restoration of protein homeostasis in an ethanol-fed mouse model of liver disease.
Methods: Studies were performed in ethanol-treated differentiated C2C12 myotubes and physiological relevance established in an ethanol-fed mouse model of alcohol-related liver disease (mALD) or pair-fed control C57BL/6 mice. Transcriptome and proteome from ethanol treated-myotubes and gastrocnemius muscle from mALD and pair-fed mice were analyzed to identify target pathways and molecules. Readouts including signaling responses and autophagy markers by immunoblots, mitochondrial oxidative function and free radical generation, and metabolic studies by gas chromatography-mass spectrometry and sarcopenic phenotype by imaging.
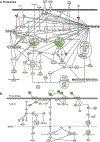
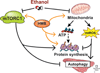
Results: Multiomics analyses showed that ethanol impaired skeletal muscle mTORC1 signaling, mitochondrial oxidative pathways, including intermediary metabolite regulatory genes, interleukin-6, and amino acid degradation pathways are β-hydroxymethyl-butyrate targets. Ethanol decreased mTORC1 signaling, increased autophagy flux, impaired mitochondrial oxidative function with decreased tricarboxylic acid cycle intermediary metabolites, ATP synthesis, protein synthesis and myotube diameter that were reversed by HMB. Consistently, skeletal muscle from mALD had decreased mTORC1 signaling, reduced fractional and total muscle protein synthesis rates, increased autophagy markers, lower intermediary metabolite concentrations, and lower muscle mass and fiber diameter that were reversed by β-hydroxymethyl-butyrate treatment.
Conclusion: An innovative multiomics approach followed by experimental validation showed that β-hydroxymethyl-butyrate restores muscle protein homeostasis in liver disease.
Keywords: Autophagy; Mitochondria; Pathway-analyses; Protein synthesis; Proteomics; Transcriptomics.
© Copyright by the Author(s). Published by Cell Physiol Biochem Press.
Conflict of interest statement
The authors have no conflicts to declare.
Figures



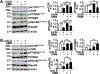

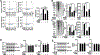
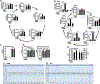


References
-
- Ferreira LLG, Andricopulo AD: ADMET modeling approaches in drug discovery. Drug Discov Today 2019;24:1157–1165. - PubMed
MeSH terms
Substances
Grants and funding
- R56 HL141744/HL/NHLBI NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- U01 DK061732/DK/NIDDK NIH HHS/United States
- T32 DK083251/DK/NIDDK NIH HHS/United States
- K12 HL141952/HL/NHLBI NIH HHS/United States
- R01 AA028190/AA/NIAAA NIH HHS/United States
- R21 AA022742/AA/NIAAA NIH HHS/United States
- U01 AA026976/AA/NIAAA NIH HHS/United States
- K08 AA028794/AA/NIAAA NIH HHS/United States
- L30 AA027927/AA/NIAAA NIH HHS/United States
- R01 DK113196/DK/NIDDK NIH HHS/United States
- P50 AA024333/AA/NIAAA NIH HHS/United States
- R01 AR057347/AR/NIAMS NIH HHS/United States
- R01 GM119174/GM/NIGMS NIH HHS/United States
- S10 OD023436/OD/NIH HHS/United States
- NIH R21 AR 071046, NIH RO1 GM 119174, NIH RO1 DK 113196, NIH RO1 AA 021890, NIH P50 AA024333, NIH UO1 AA 026976, NIH UO1 021890, NIH R56HL141744, NIH 3UO1AA026976, NIH RO1 AR057347, NIH K12HL141952/NIH (National Institutes of Health)/United States
- R21 AR071046/AR/NIAMS NIH HHS/United States
- R01 DK083414/DK/NIDDK NIH HHS/United States
- U01 AA021890/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

