Reinvigorating the Chiral Pool: Chemoenzymatic Approaches to Complex Peptides and Terpenoids
- PMID: 33543931
- PMCID: PMC8138964
- DOI: 10.1021/acs.accounts.0c00823
Reinvigorating the Chiral Pool: Chemoenzymatic Approaches to Complex Peptides and Terpenoids
Abstract
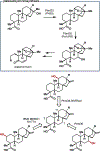
Biocatalytic transformations that leverage the selectivity and efficiency of enzymes represent powerful tools for the construction of complex natural products. Enabled by innovations in genome mining, bioinformatics, and enzyme engineering, synthetic chemists are now more than ever able to develop and employ enzymes to solve outstanding chemical problems, one of which is the reliable and facile generation of stereochemistry within natural product scaffolds. In recognition of this unmet need, our group has sought to advance novel chemoenzymatic strategies to both expand and reinvigorate the chiral pool. Broadly defined, the chiral pool comprises cheap, enantiopure feedstock chemicals that serve as popular foundations for asymmetric total synthesis. Among these building blocks, amino acids and enantiopure terpenes, whose core structures can be mapped onto several classes of structurally and pharmaceutically intriguing natural products, are of particular interest to the synthetic community.In this Account, we summarize recent efforts from our group in leveraging biocatalytic transformations to expand the chiral pool, as well as efforts toward the efficient application of these transformations in natural products total synthesis, the ultimate testing ground for any novel methodology. First, we describe several examples of enzymatic generation of noncanonical amino acids as means to simplify the synthesis of peptide natural products. By extracting amino acid hydroxylases from native biosynthetic pathways, we obtain efficient access to hydroxylated variants of proline, lysine, arginine, and their derivatives. The newly installed hydroxyl moiety then becomes a chemical handle that can facilitate additional complexity generation, thereby expanding the pool of amino acid-derived building blocks available for peptide synthesis. Next, we present our efforts in enzymatic C-H oxidations of diverse terpene scaffolds, in which traditional chemistry can be combined with strategic applications of biocatalysis to selectively and efficiently derivatize several commercial terpenoid skeletons. The synergistic logic of this approach enables a small handful of synthetic intermediates to provide access to a plethora of terpenoid natural product families. Taken together, these findings demonstrate the advantages of applying enzymes in total synthesis in conjunction with established methodologies, as well as toward the expansion of the chiral pool to enable facile incorporation of stereochemistry during synthetic campaigns.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

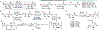
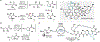

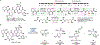


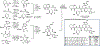


Similar articles
-
Chemoenzymatic Total Synthesis of Natural Products.Acc Chem Res. 2021 Mar 16;54(6):1374-1384. doi: 10.1021/acs.accounts.0c00810. Epub 2021 Feb 18. Acc Chem Res. 2021. PMID: 33600149 Free PMC article. Review.
-
C-C Bond Cleavage of α-Pinene Derivatives Prepared from Carvone as a General Strategy for Complex Molecule Synthesis.Acc Chem Res. 2022 Mar 1;55(5):746-758. doi: 10.1021/acs.accounts.1c00783. Epub 2022 Feb 16. Acc Chem Res. 2022. PMID: 35170951 Free PMC article.
-
Synthetic utility of oxygenases in site-selective terpenoid functionalization.J Ind Microbiol Biotechnol. 2021 Jun 4;48(3-4):kuab002. doi: 10.1093/jimb/kuab002. J Ind Microbiol Biotechnol. 2021. PMID: 33928356 Free PMC article. Review.
-
Applications of Oxygenases in the Chemoenzymatic Total Synthesis of Complex Natural Products.Biochemistry. 2018 Jan 30;57(4):403-412. doi: 10.1021/acs.biochem.7b00998. Epub 2017 Nov 29. Biochemistry. 2018. PMID: 29140086 Review.
-
Chemo-enzymatic synthesis of natural products and their analogs.Curr Opin Biotechnol. 2022 Oct;77:102759. doi: 10.1016/j.copbio.2022.102759. Epub 2022 Jul 28. Curr Opin Biotechnol. 2022. PMID: 35908314 Review.
Cited by
-
Advances, opportunities, and challenges in methods for interrogating the structure activity relationships of natural products.Nat Prod Rep. 2024 Oct 17;41(10):1543-1578. doi: 10.1039/d4np00009a. Nat Prod Rep. 2024. PMID: 38912779 Free PMC article. Review.
-
Assignment-free chirality detection in unknown samples via microwave three-wave mixing.Commun Chem. 2022 Mar 14;5(1):31. doi: 10.1038/s42004-022-00641-3. Commun Chem. 2022. PMID: 36697786 Free PMC article.
-
Oxygenating Biocatalysts for Hydroxyl Functionalisation in Drug Discovery and Development.ChemMedChem. 2022 Jun 20;17(12):e202200115. doi: 10.1002/cmdc.202200115. Epub 2022 May 2. ChemMedChem. 2022. PMID: 35385205 Free PMC article. Review.
-
Biocatalytic synthesis of peptidic natural products and related analogues.iScience. 2021 May 4;24(5):102512. doi: 10.1016/j.isci.2021.102512. eCollection 2021 May 21. iScience. 2021. PMID: 34041453 Free PMC article. Review.
-
Spheroplasts preparation boosts the catalytic potential of a squalene-hopene cyclase.Nat Commun. 2022 Oct 21;13(1):6269. doi: 10.1038/s41467-022-34030-0. Nat Commun. 2022. PMID: 36271006 Free PMC article.
References
-
-
Zwick CR III; Renata H Remote C–H Hydroxylation by an α-Ketoglutarate-Dependent Dioxygenase Enables Efficient Chemoenzymatic Synthesis of Manzacidin C and Proline Analogs. J. Am. Chem. Soc. 2018, 140, 1165–1169.
This study resulted in the functional characterization of leucine 5-hydroxylase GriE and showcased its synthetic utility in the synthesis of rare alkaloid manzacidin C and in the construction of proline derivatives.
-
-
-
Li J; Li F; King-Smith E; Renata H Merging Chemoenzymatic and Radical-Based Retrosynthetic Logic for Rapid and Modular Synthesis of Oxidized Meroterpenoids. Nat. Chem. 2020, 12, 173–179.
This work demonstrated the potential of biocatalytic C–H oxidation as an effective means to functionalize sclareol and sclareolide toward the synthesis of eight oxidized meroterpenoids from a handful of late-stage synthetic intermediates.
-
-
-
Zhang X; King-Smith E; Dong L-B; Yang L-C; Rudolf JD; Shen B; Renata H Divergent Synthesis of Complex Diterpenes Through a Hybrid Oxidative Approach. Science 2020, 369, 799–806.
This effort utilized three terpene hydroxylases to selective oxidize various polycyclic diterpenes and enable the synthesis of nine complex natural products in 10 steps or less from steviol.
-
-
-
Amatuni A; Shuster A; Adibekian A; Renata H Concise Chemoenzymatic Total Synthesis and Identification of Cellular Targets of Cepafungin I. Cell Chem. Biol. 2020, 27, 1318–1326.
This work employed biocatalytic lysine hydroxylation to enable concise synthesis of the potent proteasome inhibitor cepafungin I and analogues, which were then subjected to proteomic analysis to probe structure-activity relationships and confirm biological activity.
-
-
- Yamaguchi J; Yamaguchi AD; Itami K C–H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2012, 51, 8960–9009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

