Characterisation of a cysteine protease from poultry red mites and its potential use as a vaccine for chickens
- PMID: 33544074
- PMCID: PMC7863971
- DOI: 10.1051/parasite/2021005
Characterisation of a cysteine protease from poultry red mites and its potential use as a vaccine for chickens
Abstract
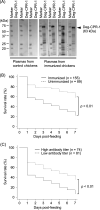
Poultry red mites (PRMs, Dermanyssus gallinae) are ectoparasites that negatively affect farmed chickens, leading to serious economic losses worldwide. Acaricides have been used to control PRMs in poultry houses. However, some PRMs have developed resistance to acaricides, and therefore different approaches are required to manage the problems caused by PRMs. Vaccination of chickens is one of the methods being considered to reduce the number of PRMs in poultry houses. In a previous study, a cysteine protease, Deg-CPR-1, was identified as a candidate vaccine against PRMs distributed in Europe. In this study, we investigated the characteristics of Deg-CPR-1. A phylogenetic analysis revealed that Deg-CPR-1 is closely related to the digestive cysteine proteases of other mite species, and it was classified into a cluster different from that of chicken cathepsins. Deg-CPR-1 of PRMs in Japan has an amino acid substitution compared with that of PRMs in Europe, but it showed efficacy as a vaccine, consistent with previous findings. Deg-CPR-1 exhibited cathepsin L-like enzyme activity. In addition, the Deg-CPR-1 mRNA was expressed in the midgut and in all stages of PRMs that feed on blood. These results imply that Deg-CPR-1 in the midgut may have important functions in physiological processes, and the inhibition of its expression may contribute to the efficacy of a Deg-CPR-1-based vaccine. Further research is required to fully understand the mechanisms of vaccine efficacy.
Title: Caractérisation d’une cystéine protéase des poux rouges de la volaille et son utilisation potentielle comme vaccin pour les poulets.
Abstract: Les acariens communément appelés poux rouges de la volaille (PRV, Dermanyssus gallinae) sont des ectoparasites qui affectent négativement les poulets d’élevage, entraînant de graves pertes économiques au niveau mondial. Des acaricides ont été utilisés pour contrôler les PRV dans les poulaillers. Cependant, certains PRV ont développé une résistance aux acaricides, et par conséquent, différentes approches sont nécessaires pour gérer les problèmes qu’ils causent. La vaccination des poulets est l’une des méthodes envisagées pour réduire le nombre de PRV dans les poulaillers. Dans une étude précédente, une cystéine protéase, Deg-CPR-1, a été identifiée comme un vaccin candidat contre les PRV distribués en Europe. Dans cette étude, nous avons étudié les caractéristiques de Deg-CPR-1. L’analyse phylogénétique a révélé que Deg-CPR-1 est étroitement liée aux cystéine protéases digestives d’autres espèces d’acariens, et elle a été classée dans un groupe différent de celui des cathepsines de poulet. La Deg-CPR-1 des PRV au Japon a une substitution d’acide aminé par rapport à celle des PRV en Europe, mais elle a montré une efficacité en tant que vaccin, conformément aux résultats précédents. Deg-CPR-1 a présenté une activité enzymatique de type cathepsine L. De plus, l’ARNm de Deg-CPR-1 était exprimé dans l’intestin moyen et à tous les stades où les PRV se nourrissent de sang. Ces résultats impliquent que Deg-CPR-1 dans l’intestin moyen peut avoir des fonctions importantes dans les processus physiologiques, et que l’inhibition de son expression peut contribuer à l’efficacité d’un vaccin basé sur Deg-CPR-1. Des recherches supplémentaires sont nécessaires pour comprendre pleinement les mécanismes de l’efficacité du vaccin.
Keywords: Cathepsin L; Cysteine protease; Deg-CPR-1; Dermanyssus gallinae; Poultry red mite; Vaccine candidate.
© S. Murata et al., published by EDP Sciences, 2021.
Figures



References
-
- Bartley K, Huntley JF, Wright HW, Nath M, Nisbet AJ. 2012. Assessment of cathepsin D and L-like proteinases of poultry red mite, Dermanyssus gallinae (De Geer), as potential vaccine antigens. Parasitology, 139, 755–765. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
