Change in Fibrosis 4 Index as Predictor of High Risk of Incident Hepatocellular Carcinoma After Eradication of Hepatitis C Virus
- PMID: 33544129
- PMCID: PMC8824825
- DOI: 10.1093/cid/ciaa1307
Change in Fibrosis 4 Index as Predictor of High Risk of Incident Hepatocellular Carcinoma After Eradication of Hepatitis C Virus
Abstract
Background: It is unclear whether the fibrosis 4 index (FIB-4), a marker of liver fibrosis, at baseline and change in FIB-4 after sustained virological response (SVR) is associated with incident hepatocellular carcinoma (HCC) risk. In this study, we examined the association of incident HCC risk with baseline FIB-4 and sustained high FIB-4 (>3.25) at any time point after SVR.
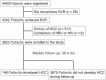
Methods: A total of 3823 patients who received direct-acting antiviral treatment and achieved SVR were enrolled. The FIB-4 was measured 24 weeks after the end of direct-acting antiviral treatment and achievement of SVR (SVR24), and 1, 2, and 3 years after SVR24, after which subsequent HCC development was investigated.
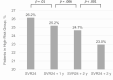
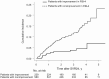
Results: In patients with an FIB-4 >3.25 at SVR24 and 1, 2, and 3 years after SVR24, subsequent HCC development was significantly higher than in those with an FIB-4 ≤3.25 at each point. The rates of HCC development 1, 2, 3, and 4 years after SVR24 were significantly higher in patients with sustained FIB-4 >3.25 than in those whose FIB-4 decreased to ≤3.25 (5.4%, 9.2%, 11.7%, and 16.0%, respectively, vs 2.2%, 3.1%, 3.7%, and 4.4%; P < .001). The adjusted hazard ratios (95% confidence intervals) for an FIB-4 >3.25 at SVR24 and 1, 2, and 3 years later were 3.38 (2.4-4.8), 2.95 (1.9-4.7), 2.62 (1.3-5.1), and 3.37 (1.4-9.8), respectively.
Conclusions: The FIB-4 could be used to assess HCC development risk at any time after SVR, and changes in FIB-4 were associated with changes in the HCC development risk. Repeated assessments of FIB-4 could serve as a prognostic indicator of a high-risk HCC cohort that may require more intensive HCC surveillance strategy.
Keywords: DAA; FIB-4; SVR; hepatocellular carcinoma.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Afdhal N, Reddy KR, Nelson DR, et al. ; ION-2 Investigators . Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–93. - PubMed
-
- Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015; 15:645–53. - PubMed
-
- Akahane T, Kurosaki M, Itakura J, et al. Real-world efficacy and safety of sofosbuvir + ribavirin for hepatitis C genotype 2: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. Hepatol Res 2019; 49:264–70. - PubMed
-
- Kusakabe A, Kurosaki M, Itakura J, et al. Efficacy and safety of glecaprevir/pibrentasvir as retreatment therapy for patients with genotype 2 chronic hepatitis C who failed prior sofosbuvir plus ribavirin regimen. Hepatol Res 2019; 49:1121–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

