Onset, Maintenance, and Cessation of Effect of Galcanezumab for Prevention of Migraine: A Narrative Review of Three Randomized Placebo-Controlled Trials
- PMID: 33544305
- PMCID: PMC7932975
- DOI: 10.1007/s12325-021-01632-x
Onset, Maintenance, and Cessation of Effect of Galcanezumab for Prevention of Migraine: A Narrative Review of Three Randomized Placebo-Controlled Trials
Abstract
Introduction: Galcanezumab, a humanized monoclonal antibody that binds to calcitonin gene-related peptide, is approved for the preventive treatment of migraine in adults. It is self-administered once monthly as a subcutaneous injection. This paper describes the time course of effect of galcanezumab in patients with episodic and chronic migraine.
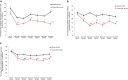
Methods: Data were based on three double-blind, placebo-controlled, phase 3 studies. Patients (1773 episodic and 1113 chronic) were randomized (2:1:1) to monthly doses of placebo, galcanezumab 120 mg with a 240 mg loading dose, or galcanezumab 240 mg (January 2016-March 2017). Onset of effect was determined using a sequential analysis approach based on earliest time point at which galcanezumab achieved and subsequently maintained statistical superiority to placebo. Maintenance of effect was a comparison of the percentages of galcanezumab- and placebo-treated patients with maintenance of at least 50% response at the individual patient level. Cessation of effect was determined during a 4-month post-treatment period on the basis of change from baseline in monthly migraine headache days.
Results: Galcanezumab led to a lower percentage of patients who had a migraine headache on the first day after injection, provided maintenance of effect throughout the duration of the double-blind treatment period, and gradually lost effect without signs of rebound headache throughout the post-treatment period in most patients with episodic and chronic migraine.
Conclusion: Galcanezumab is a novel preventive therapeutic option for adult patients with migraine that has early onset of action, maintenance of effect, and gradual reduction of effect upon treatment cessation.
Trial registration: ClinicalTrials.gov: NCT02614183 (EVOLVE-1); NCT02614196 (EVOLVE-2); NCT02614261 (REGAIN).
Keywords: CGRP antagonist; Cessation; Galcanezumab; Maintenance; Migraine; Migraine prevention; Onset.
Figures



References
-
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. - PubMed
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

