Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility
- PMID: 33544411
- PMCID: PMC8357003
- DOI: 10.1002/hbm.25330
Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility
Abstract
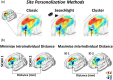
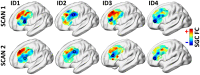
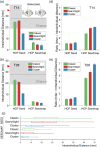
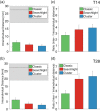
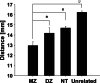
Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) is an established treatment for refractory depression, however, therapeutic outcomes vary. Mounting evidence suggests that clinical response relates to functional connectivity with the subgenual cingulate cortex (SGC) at the precise DLPFC stimulation site. Critically, SGC-related network architecture shows considerable interindividual variation across the spatial extent of the DLPFC, indicating that connectivity-based target personalization could potentially be necessary to improve treatment outcomes. However, to date accurate personalization has not appeared feasible, with recent work indicating that the intraindividual reproducibility of optimal targets is limited to 3.5 cm. Here we developed reliable and accurate methodologies to compute individualized connectivity-guided stimulation targets. In resting-state functional MRI scans acquired across 1,000 healthy adults, we demonstrate that, using this approach, personalized targets can be reliably and robustly pinpointed, with a median accuracy of ~2 mm between scans repeated across separate days. These targets remained highly stable, even after 1 year, with a median intraindividual distance between coordinates of only 2.7 mm. Interindividual spatial variation in personalized targets exceeded intraindividual variation by a factor of up to 6.85, suggesting that personalized targets did not trivially converge to a group-average site. Moreover, personalized targets were heritable, suggesting that connectivity-guided rTMS personalization is stable over time and under genetic control. This computational framework provides capacity for personalized connectivity-guided TMS targets to be robustly computed with high precision and has the flexibly to advance research in other basic research and clinical applications.
Keywords: connectivity; depression; neuroimaging; personalization; precision psychiatry; transcranial magnetic stimulation.
© 2021 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors report no competing interests.
Figures


Comment in
-
Individualized functional targeting for rTMS: A powerful idea whose time has come?Hum Brain Mapp. 2021 Sep;42(13):4079-4080. doi: 10.1002/hbm.25543. Epub 2021 May 25. Hum Brain Mapp. 2021. PMID: 34032348 Free PMC article. No abstract available.
References
-
- Barbour, T., Lee, E., Ellard, K., & Camprodon, J. (2019). Individualized TMS target selection for MDD: Clinical outcomes, mechanisms of action and predictors of response. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 12(2), 516.
-
- Berlim, M. T., van den Eynde, F., Tovar‐Perdomo, S., & Daskalakis, Z. J. (2014). Response, remission and drop‐out rates following high‐frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: A systematic review and meta‐analysis of randomized, double‐blind and sham‐controlled trials. Psychological Medicine, 44(2), 225–239.S0033291713000512. 10.1017/S0033291713000512 - DOI - PubMed
-
- Blumberger, D. M., Vila‐Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., … Downar, J. (2018). Effectiveness of theta burst versus high‐frequency repetitive transcranial magnetic stimulation in patients with depression (THREE‐D): A randomised non‐inferiority trial. Lancet, 391(10131), 1683–1692. 10.1016/S0140-6736(18)30295-2 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

