Magnetic resonance imaging in the evaluation of pathologic nipple discharge: indications and imaging findings
- PMID: 33544650
- PMCID: PMC8010557
- DOI: 10.1259/bjr.20201013
Magnetic resonance imaging in the evaluation of pathologic nipple discharge: indications and imaging findings
Abstract
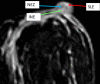
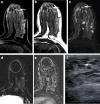
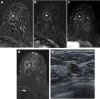
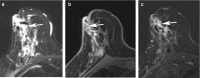
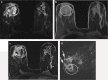
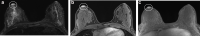
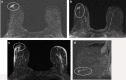
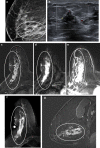
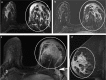
Pathologic nipple discharge (PND) is typically unilateral, spontaneous, involves a single duct, and is serous or bloody in appearance. In patients with PND, breast MRI can be helpful as an additional diagnostic tool when conventional imaging with mammogram and ultrasound are negative. MRI is able to detect the etiology of nipple discharge in 56-61% of cases when initial imaging with mammogram and ultrasound are negative. Advantages to using MRI in evaluation of PND include good visualization of the retroareolar breast and better evaluation of posterior lesions which may not be well evaluated on mammograms and galactograms. It is also less invasive compared to central duct excision. Papillomas and nipple adenomas are benign breast masses that can cause PND and are well visualized on MRI. Ductal ectasia, and infectious etiologies such as mastitis, abscess, and fistulas are additional benign causes of PND that are well evaluated with MRI. MRI is also excellent for evaluation of malignant causes of PND including Paget's disease, ductal carcinoma in-situ and invasive carcinoma. MRI's high negative predictive value of 87-98.2% is helpful in excluding malignant etiologies of PND.
Figures
References
-
- ACoR. Acrpractice parameterfor the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. 2018. Available from: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Contrast-Br....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

