Distinct clonal lineages and within-host diversification shape invasive Staphylococcus epidermidis populations
- PMID: 33544760
- PMCID: PMC7891712
- DOI: 10.1371/journal.ppat.1009304
Distinct clonal lineages and within-host diversification shape invasive Staphylococcus epidermidis populations
Abstract
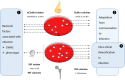
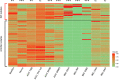
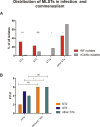
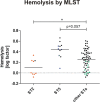
S. epidermidis is a substantial component of the human skin microbiota, but also one of the major causes of nosocomial infection in the context of implanted medical devices. We here aimed to advance the understanding of S. epidermidis genotypes and phenotypes conducive to infection establishment. Furthermore, we investigate the adaptation of individual clonal lines to the infection lifestyle based on the detailed analysis of individual S. epidermidis populations of 23 patients suffering from prosthetic joint infection. Analysis of invasive and colonizing S. epidermidis provided evidence that invasive S. epidermidis are characterized by infection-supporting phenotypes (e.g. increased biofilm formation, growth in nutrient poor media and antibiotic resistance), as well as specific genetic traits. The discriminating gene loci were almost exclusively assigned to the mobilome. Here, in addition to IS256 and SCCmec, chromosomally integrated phages was identified for the first time. These phenotypic and genotypic features were more likely present in isolates belonging to sequence type (ST) 2. By comparing seven patient-matched nasal and invasive S. epidermidis isolates belonging to identical genetic lineages, infection-associated phenotypic and genotypic changes were documented. Besides increased biofilm production, the invasive isolates were characterized by better growth in nutrient-poor media and reduced hemolysis. By examining several colonies grown in parallel from each infection, evidence for genetic within-host population heterogeneity was obtained. Importantly, subpopulations carrying IS insertions in agrC, mutations in the acetate kinase (AckA) and deletions in the SCCmec element emerged in several infections. In summary, these results shed light on the multifactorial processes of infection adaptation and demonstrate how S. epidermidis is able to flexibly repurpose and edit factors important for colonization to facilitate survival in hostile infection environments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

