Residual immune response towards decellularized homografts may be highly individual
- PMID: 33544830
- PMCID: PMC8083949
- DOI: 10.1093/ejcts/ezaa393
Residual immune response towards decellularized homografts may be highly individual
Abstract
Objectives: Decellularized homograft valves (DHVs) have shown promising clinical results, particularly in the treatment of congenital heart disease. However, DHV appears to elicit an immune response in a subset of young patients, indicated by early valve degeneration. As the decellularization process is quality controlled for each DHV, we hypothesized that there may be residual immunogenicity within the extracellular matrix of DHV.
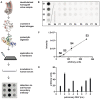
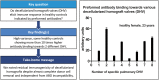
Methods: A semi-quantitative dot blot analysis was established to screen for preformed recipient antibodies using secondary anti-human antibodies. Fifteen DHV samples (7 aortic, 8 pulmonary) were solubilized and exposed to serum from 20 healthy controls.
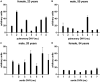
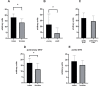
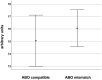
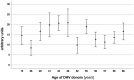
Results: The sera from young controls (n = 10, 18-25 years) showed significantly stronger binding of preformed antibodies than sera from older individuals (n = 10, 48-73 years). The difference between the means of arbitrary units was 15.1 ± 6.5 (P = 0.0315). There was high intraindividual variance in the mean amounts of arbitrary units of antibody binding with some healthy controls showing >10 times higher antibody binding towards 2 different DHV. The amount of preformed antibodies bound to DHVs was higher in aortic than in pulmonary DHVs. The mean number of antibody binding (in arbitrary units) was 17.2 ± 4.5 in aortic and 14.5 ± 4.7 in pulmonary DHV (P = 0.27). The amount of preformed antibodies bound to pulmonary DHVs was statistically significantly higher in the sera of healthy males (n = 10) than in the sera of healthy females (n = 10). The mean number of arbitrary units was 17.2 ± 4.2 in male and 11.7 ± 5.3 in female sera (P = 0.036). Antibody binding to aortic DHV was also higher in males, but not significant (18.8 ± 5.0 vs 15.6 ± 4.0). Blood group (ABO) incompatibility between the serum from controls and DHV showed no impact on antibody binding, and there was no age-related impact among DHV donors.
Conclusions: Residual immunogenicity of decellularized homografts appears to exist despite almost complete cell removal. The established dot blot method allows a semi-quantitative assessment of the individual immune response towards extracellular DHV components and potentially the possibility of preoperative homograft matching.
Keywords: Decellularization; Heart valve replacement; Homograft.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures


References
-
- Nachlas ALY, Li S, Davis ME.. Developing a clinically relevant tissue engineered heart valve—a review of current approaches. Adv Healthc Mater 2017;6: 1–30. - PubMed
-
- Emmert MY, Fioretta ES, Hoerstrup SP.. Translational challenges in cardiovascular tissue engineering. J Cardiovasc Trans Res 2017;10:139–49. - PubMed
-
- Emmert MY, Schmitt BA, Loerakker S, Sanders B, Spriestersbach H, Fioretta ES. et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med 2018;10: 1–13. - PubMed
-
- Cebotari S, Lichtenberg A, Tudorache I, Hilfiker A, Mertsching H, Leyh R. et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation 2006;114:I-132–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

