Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine
- PMID: 33544838
- PMCID: PMC7871457
- DOI: 10.1084/jem.20202486
Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine
Abstract
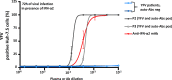
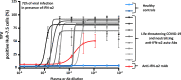
Yellow fever virus (YFV) live attenuated vaccine can, in rare cases, cause life-threatening disease, typically in patients with no previous history of severe viral illness. Autosomal recessive (AR) complete IFNAR1 deficiency was reported in one 12-yr-old patient. Here, we studied seven other previously healthy patients aged 13 to 80 yr with unexplained life-threatening YFV vaccine-associated disease. One 13-yr-old patient had AR complete IFNAR2 deficiency. Three other patients vaccinated at the ages of 47, 57, and 64 yr had high titers of circulating auto-Abs against at least 14 of the 17 individual type I IFNs. These antibodies were recently shown to underlie at least 10% of cases of life-threatening COVID-19 pneumonia. The auto-Abs were neutralizing in vitro, blocking the protective effect of IFN-α2 against YFV vaccine strains. AR IFNAR1 or IFNAR2 deficiency and neutralizing auto-Abs against type I IFNs thus accounted for more than half the cases of life-threatening YFV vaccine-associated disease studied here. Previously healthy subjects could be tested for both predispositions before anti-YFV vaccination.
© 2021 Bastard et al.
Conflict of interest statement
Disclosures: A. Homma reported, "Our institution is a non-profit producer of the yellow fever vaccine. We are public institution, part of our Ministry of Health, and provide vaccine only for National Immunization Program and UNICEF, PAHO Revolving Fund, GAVI, and WHO. We are very much interested to know all relevant scientific issues involved with our vaccine." J.L. Casanova reported a patent to application number 63/055,155, filed July 22, 2020 pending. No other disclosures were reported.
Figures



References
-
- Bousfiha, A., Jeddane L., Picard C., Al-Herz W., Ailal F., Chatila T., Cunningham-Rundles C., Etzioni A., Franco J.L., Holland S.M., et al. 2020. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J. Clin. Immunol. 40:66–81. 10.1007/s10875-020-00758-x - DOI - PMC - PubMed
-
- Bravo García-Morato, M., Calvo Apalategi A., Bravo-Gallego L.Y., Blázquez Moreno A., Simón-Fuentes M., Garmendia J.V., Méndez Echevarría A., Del Rosal Rabes T., Domínguez-Soto Á., López-Granados E., et al. 2019. Impaired control of multiple viral infections in a family with complete IRF9 deficiency. J. Allergy Clin. Immunol. 144:309–312.e10. 10.1016/j.jaci.2019.02.019 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

