The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies
- PMID: 33544940
- PMCID: PMC8248430
- DOI: 10.1111/ejh.13595
The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies
Abstract
Objectives: To estimate the risk of thrombocytopenia in various cancers and chemotherapy regimens.
Methods: Structured patient-level data from the Flatiron Health Electronic Health Record database were used to identify adult patients who received chemotherapy for a solid tumor or hematologic malignancy from 2012 to 2017. Three-month cumulative incidence of thrombocytopenia was assessed based on platelet counts, overall and by grade of thrombocytopenia. Co-occurrence of anemia, neutropenia, and leukopenia was evaluated.
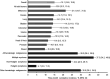
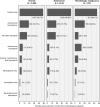
Results: Of 15,521 patients with solid tumors, 13% had thrombocytopenia within 3 months (platelet count < 100 × 109 /L); 4% had grade 3 (25 to < 50 × 109 /L), and 2% grade 4 (<25 × 109 /L) thrombocytopenia. Of 2537 patients with hematologic malignancies, 28% had any thrombocytopenia, 16% with grade 3, and 12% with grade 4. Among patients with thrombocytopenia, it occurred without another cytopenia in 18% of solid tumors and 7% of hematologic malignancies.
Conclusions: In a large, US-representative sample of patients undergoing chemotherapy in clinical practice, thrombocytopenia incidence varied across tumor and regimen types. Despite recommendations to alter chemotherapy to avoid severe thrombocytopenia, 4% of patients with solid tumors and 16% with hematologic malignancies experienced grade 3 thrombocytopenia. Prediction and prevention of thrombocytopenia may help oncologists avoid dose modifications and their adverse effects on survival.
Keywords: CIT; Thrombocytopenia; adverse event; chemotherapy; chemotherapy-induced thrombocytopenia; hematologic malignancy; incidence; solid tumor; toxicity.
© 2021 The Authors. European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
JS, CN, AM, and JP are Amgen employees and have stock ownership in Amgen Inc; GS is and has been a clinical investigator for Amgen and trials.
Figures

References
-
- Hitron A, Steinke D, Sutphin S, Lawson A, Talbert J, Adams V. Incidence and risk factors of clinically significant chemotherapy‐induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract. 2011;17(4):312‐319. - PubMed
-
- ten Berg MJ, van den Bemt PMLA, Shantakumar S, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy results from a retrospective hospital‐based cohort. Drug Saf. 2011;34(12):1151‐1160. - PubMed
-
- Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000–2007. Clin Ther. 2009;31(Pt 2):2416‐2432. - PubMed
-
- Goldberg GL, Gibbon DG, Smith HO, DeVictoria C, Runowicz CD, Burns ER. Clinical impact of chemotherapy‐induced thrombocytopenia in patients with gynecologic cancer. J Clin Oncol. 1994;12(11):2317‐2320. - PubMed
-
- Kantarjian H, Giles F, List A, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007;109(9):1705‐1714. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

