Coronavirus (SARS-CoV-2): a systematic review for potential vaccines
- PMID: 33545014
- PMCID: PMC8920137
- DOI: 10.1080/21645515.2020.1865774
Coronavirus (SARS-CoV-2): a systematic review for potential vaccines
Abstract
COVID-19 is an international public health emergency in need of effective and safe vaccines for SARS-CoV-2. A systematic review has been done to analyze the availability, development and status of new COVID-19 vaccine candidates as well as the status of vaccines for other diseases that might be effective against SARS-CoV-2 infection. PubMed, MEDLINE, EMBASE, Science Direct, Google Scholar, Cochrane library, ClinicalTrials.gov, Web of Science and different trial registries were searched for currently available and probable future vaccines. Articles and ongoing clinical trials are included to ascertain the availability and developmental approaches of new vaccines that could limit the present and future outbreaks. Pharmaceutical companies and institutions are at different stages of developing new vaccines, and extensive studies and clinical trials are still required.
Keywords: SARS-CoV-2; clinical trial; coronavirus; covid-19; vaccine candidates; vaccine development.
Figures
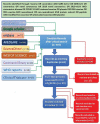

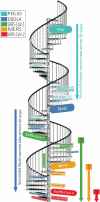
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
