A randomized phase 1 study of the safety and immunogenicity of 2 novel pneumococcal conjugate vaccines in healthy Japanese adults in the United States
- PMID: 33545022
- PMCID: PMC8189073
- DOI: 10.1080/21645515.2020.1863177
A randomized phase 1 study of the safety and immunogenicity of 2 novel pneumococcal conjugate vaccines in healthy Japanese adults in the United States
Abstract
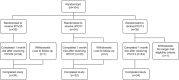
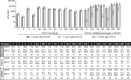
Expanding serotype coverage of pneumococcal conjugate vaccines (PCVs) to target prevailing disease-causing serotypes could further reduce disease burden. To address this need, 2 different PCVs have been investigated: a 20-valent PCV (PCV20; includes the 13 serotypes in the 13-valent PCV [PCV13] plus 7 additional serotypes [8, 10A, 11A, 12F, 15B, 22F, 33F]) and a complementary 7-valent PCV (cPCV7; contains only the 7 additional serotypes). This phase 1b, randomized, controlled, double-blind study evaluated PCV20 and cPCV7 safety and immunogenicity in healthy Japanese adults 18-49 years of age residing in the United States for ≤5 years. Participants (n = 104) were randomized equally to receive a single dose of PCV20, cPCV7, or PCV13. Immunogenicity was assessed at baseline and 1 month after vaccination using serotype-specific opsonophagocytic activity (OPA) titers and serotype-specific immunoglobulin G (IgG) concentrations. Prompted local reactions and systemic events; adverse events (AEs); and serious AEs and newly diagnosed chronic disease were assessed 14 days, through 1 month, and upto 6 months following vaccination, respectively. OPA immune responses were robust for all 20 serotypes in the PCV20 group and for the 7 serotypes in the cPCV7 group 1 month after vaccination. IgG immune response showed similar trends. Injection site pain and muscle pain were the most common local reaction and systemic event; the majority were mild or moderate in severity. Few AEs and no severe AEs, serious AEs, or safety-related withdrawals were reported. Taken together, administration of PCV20 or cPCV7 in Japanese adults was well tolerated and induced robust serotype-specific functional immune responses. NCT03642847.
Keywords: PCV20; Streptococcus pneumoniae; cPCV7; clinical trial; immunogenicity; pneumococcal conjugate vaccine; safety.
Figures

References
-
- World Health Organization . 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec. 2008;83(42):373–84. - PubMed
-
- Collaborators GBDLRI . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. doi:10.1016/S1473-3099(18)30310-4. - DOI - PMC - PubMed
-
- World Health Organization . Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec. 2012;87(14):129–44. - PubMed
-
- National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division . Pneumococcal infections in 2017. Japan: Ministry of Health, Labour and Welfare of Japan; 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
