Melanoma models for the next generation of therapies
- PMID: 33545064
- PMCID: PMC8378471
- DOI: 10.1016/j.ccell.2021.01.011
Melanoma models for the next generation of therapies
Abstract
There is a lack of appropriate melanoma models that can be used to evaluate the efficacy of novel therapeutic modalities. Here, we discuss the current state of the art of melanoma models including genetically engineered mouse, patient-derived xenograft, zebrafish, and ex vivo and in vitro models. We also identify five major challenges that can be addressed using such models, including metastasis and tumor dormancy, drug resistance, the melanoma immune response, and the impact of aging and environmental exposures on melanoma progression and drug resistance. Additionally, we discuss the opportunity for building models for rare subtypes of melanomas, which represent an unmet critical need. Finally, we identify key recommendations for melanoma models that may improve accuracy of preclinical testing and predict efficacy in clinical trials, to help usher in the next generation of melanoma therapies.
Keywords: animal models; drug discovery; immunotherapy; melanoma; targeted therapy; therapeutics; tumor microenvironment.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.J.A. is a paid consultant for Microbiotica and receives grant support from AstraZeneca and OpenTargets. N.A. is a consultant for Viela Bio. A.E.A. reports receiving a commercial research grant from Pfizer. (2013–2017) and has ownership interest in patent number 9880150. M.B. is a consultant for Eli Lilly and Company and Bristol-Myers Squibb. P.C. received grants and personal fees from Deciphera, personal fees from Exelixis, grants from Array/Pfizer, and personal fees from Zai lab. S. Khan has a US patent pending (62/654,025). S. Kobold received research support from TCR2 Inc and Arcus Bioscience for work unrelated to this manuscript, has licensed IP to TCR2 Inc, has received consultancy fees from TCR2 Inc and Novartis, and is an inventor of several patents and patent applications in the field of cancer immunotherapy. R.M. consults for Pfizer, and as a former employee of the Institute of Cancer Research he may benefit financially from drug-discovery programs that are commercialized. M.M. receives research funding from Pfizer and Deciphera Pharma; serves as a scientific advisor to Pfizer, Deciphera Pharma, Revolution Medicine, and ARO; and receives royalty income from the University of California for Braf(CA) mice, which are the basis of several GEM models of BRAF(V600E)-driven melanoma described in this review. Z.A.R. is founder and scientific advisor of Pangea Therapeutics. Y.S. is a consultant of Achilles Therapeutics Limited. A.T.W. sits on the advisory board for Melanoma Research Foundation, Phoremost Technologies, and Healthe Scientific. R.M.W. is a paid consultant to N-of-One Therapeutics, a subsidiary of Qiagen; is on the Scientific Advisory Board of Consano but receives no income for this; and receives royalty payments for the use of the casper line from Carolina Biologicals. L.I.Z. is a founder and stockholder of Fate Therapeutics, CAMP4 Therapeutics, and Scholar Rock, and a consultant for Celularity.
Figures
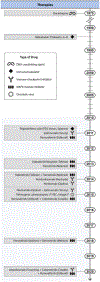

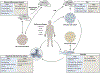
References
-
- Alicea GM, Rebecca VW, Goldman AR, Fane ME, Douglass SM, Behera R, Webster MR, Kugel CH 3rd, Ecker BL, Caino MC, et al. (2020). Changes in aged fibroblast lipid metabolism induce age-dependent melanoma cell resistance to targeted therapy via the fatty acid transporter FATP2. Cancer Discov. 10, 1282–1295. - PMC - PubMed
-
- Alpert A, Moore LS, Dubovik T, and Shen-Orr SS (2018). Alignment of single-cell trajectories to compare cellular expression dynamics. Nat. Methods 15, 267–270. - PubMed
Publication types
MeSH terms
Grants and funding
- K22 CA197058/CA/NCI NIH HHS/United States
- R21 ES032305/ES/NIEHS NIH HHS/United States
- A22902/CRUK_/Cancer Research UK/United Kingdom
- P30 CA010815/CA/NCI NIH HHS/United States
- P01 CA163222/CA/NCI NIH HHS/United States
- P50 CA225450/CA/NCI NIH HHS/United States
- P01 CA206980/CA/NCI NIH HHS/United States
- R13 CA250294/CA/NCI NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
- R01 CA244660/CA/NCI NIH HHS/United States
- A27412/CRUK_/Cancer Research UK/United Kingdom
- P01 CA128814/CA/NCI NIH HHS/United States
- 17240/CRUK_/Cancer Research UK/United Kingdom
- R01 CA196660/CA/NCI NIH HHS/United States
- R01 CA196278/CA/NCI NIH HHS/United States
- R01 CA215733/CA/NCI NIH HHS/United States
- R35 CA197465/CA/NCI NIH HHS/United States
- R01 CA229215/CA/NCI NIH HHS/United States
- DP2 CA186572/CA/NCI NIH HHS/United States
- R01 CA238237/CA/NCI NIH HHS/United States
- R01 CA182635/CA/NCI NIH HHS/United States
- P01 CA114046/CA/NCI NIH HHS/United States
- R01 CA176839/CA/NCI NIH HHS/United States
- R01 CA103846/CA/NCI NIH HHS/United States
- MR/S01473X/1/MRC_/Medical Research Council/United Kingdom
- R01 CA228216/CA/NCI NIH HHS/United States
- R01 CA212376/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 CA232256/CA/NCI NIH HHS/United States
- R01 CA216101/CA/NCI NIH HHS/United States
- R01 CA216846/CA/NCI NIH HHS/United States
- R01 CA253977/CA/NCI NIH HHS/United States
- U01 CA233096/CA/NCI NIH HHS/United States
- R01 CA238317/CA/NCI NIH HHS/United States
- R01 GM101171/GM/NIGMS NIH HHS/United States
- R01 CA204002/CA/NCI NIH HHS/United States
- R03 CA227349/CA/NCI NIH HHS/United States
- 19279/CRUK_/Cancer Research UK/United Kingdom
- R01 CA207935/CA/NCI NIH HHS/United States
- P50 CA217694/CA/NCI NIH HHS/United States
- P50 CA121974/CA/NCI NIH HHS/United States
- R37 CA240914/CA/NCI NIH HHS/United States
- R01 AR070234/AR/NIAMS NIH HHS/United States
- R01 CA238163/CA/NCI NIH HHS/United States
- R01 CA232246/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U54 CA224070/CA/NCI NIH HHS/United States
- U01 CA238728/CA/NCI NIH HHS/United States
- 22902/CRUK_/Cancer Research UK/United Kingdom
- R01 CA226888/CA/NCI NIH HHS/United States
- R01 CA121118/CA/NCI NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
- 100282/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- R01 CA240633/CA/NCI NIH HHS/United States
- MC_UU_00007/9/MRC_/Medical Research Council/United Kingdom
- R01 GM071725/GM/NIGMS NIH HHS/United States
- C5759/A29061/CRUK_/Cancer Research UK/United Kingdom
- R01 CA237213/CA/NCI NIH HHS/United States
- DP2 CA174499/CA/NCI NIH HHS/United States
- MC_UU_00007/9/MRC_/Medical Research Council/United Kingdom
- R01 CA196566/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

