Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01
- PMID: 33545080
- PMCID: PMC7903922
- DOI: 10.1016/j.stem.2021.01.004
Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01
Abstract
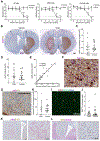
Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra leading to disabling deficits. Dopamine neuron grafts may provide a significant therapeutic advance over current therapies. We have generated midbrain dopamine neurons from human embryonic stem cells and manufactured large-scale cryopreserved dopamine progenitors for clinical use. After optimizing cell survival and phenotypes in short-term studies, the cell product, MSK-DA01, was subjected to an extensive set of biodistribution, toxicity, and tumorigenicity assessments in mice under GLP conditions. A large-scale efficacy study was also performed in rats with the same lot of cells intended for potential human use and demonstrated survival of the grafted cells and behavioral amelioration in 6-hydroxydopamine lesioned rats. There were no adverse effects attributable to the grafted cells, no obvious distribution outside the brain, and no cell overgrowth or tumor formation, thus paving the way for a future clinical trial.
Keywords: GMP; Parkinson’s disease; cell therapy; dopamine neurons; human embryonic stem cells; human pluripotent stem cells; preclinical study; safety studies; transplantation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests V.T. and L.S. are scientific co-founders and consultants of BlueRock Therapeutics. M.T., S.I., and S.M. are currently employed at BlueRock Therapeutics Inc. Other authors declare no competing interests. MSK-DA01 is the subject of a patent, US2018/0094242 A1, filed by the Memorial Sloan Kettering Cancer Center.
Figures

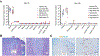


Comment in
-
Clinical Trial for Parkinson's Disease Gets a Green Light in the US.Cell Stem Cell. 2021 Feb 4;28(2):182-183. doi: 10.1016/j.stem.2021.01.013. Cell Stem Cell. 2021. PMID: 33545076
-
Embryonic stem cells go from bench to bedside for Parkinson's disease.Cell Rep Med. 2021 Apr 20;2(4):100251. doi: 10.1016/j.xcrm.2021.100251. eCollection 2021 Apr 20. Cell Rep Med. 2021. PMID: 33948581 Free PMC article.
References
-
- Barker RA, Farrell K, Guzman NV, He X, Lazic SE, Moore S, Morris R, Tyers P, Wijeyekoon R, Daft D, et al. (2019). Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med 25, 1045–1053. - PubMed
-
- Cyranoski D (2018). ‘Reprogrammed’ stem cells implanted into patient with Parkinson’s disease. Nat. News https://www.nature.com/articles/d41586-018-07407-9.
-
- Freed CR, Greene PE, Breeze RE, Tsai W-Y, DuMouchel W, Kao R, Dillion S, Winifield H, Culver S, Trojanowsk JQ, et al. (2001). Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med 344, 710–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

