Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia
- PMID: 33545094
- PMCID: PMC7852454
- DOI: 10.1016/S0140-6736(21)00234-8
Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia
Erratum in
-
Department of Error.Lancet. 2021 Feb 20;397(10275):670. doi: 10.1016/S0140-6736(21)00386-X. Lancet. 2021. PMID: 33610213 Free PMC article. No abstract available.
Abstract
Background: A heterologous recombinant adenovirus (rAd)-based vaccine, Gam-COVID-Vac (Sputnik V), showed a good safety profile and induced strong humoral and cellular immune responses in participants in phase 1/2 clinical trials. Here, we report preliminary results on the efficacy and safety of Gam-COVID-Vac from the interim analysis of this phase 3 trial.
Methods: We did a randomised, double-blind, placebo-controlled, phase 3 trial at 25 hospitals and polyclinics in Moscow, Russia. We included participants aged at least 18 years, with negative SARS-CoV-2 PCR and IgG and IgM tests, no infectious diseases in the 14 days before enrolment, and no other vaccinations in the 30 days before enrolment. Participants were randomly assigned (3:1) to receive vaccine or placebo, with stratification by age group. Investigators, participants, and all study staff were masked to group assignment. The vaccine was administered (0·5 mL/dose) intramuscularly in a prime-boost regimen: a 21-day interval between the first dose (rAd26) and the second dose (rAd5), both vectors carrying the gene for the full-length SARS-CoV-2 glycoprotein S. The primary outcome was the proportion of participants with PCR-confirmed COVID-19 from day 21 after receiving the first dose. All analyses excluded participants with protocol violations: the primary outcome was assessed in participants who had received two doses of vaccine or placebo, serious adverse events were assessed in all participants who had received at least one dose at the time of database lock, and rare adverse events were assessed in all participants who had received two doses and for whom all available data were verified in the case report form at the time of database lock. The trial is registered at ClinicalTrials.gov (NCT04530396).
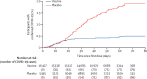
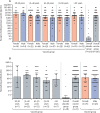
Findings: Between Sept 7 and Nov 24, 2020, 21 977 adults were randomly assigned to the vaccine group (n=16 501) or the placebo group (n=5476). 19 866 received two doses of vaccine or placebo and were included in the primary outcome analysis. From 21 days after the first dose of vaccine (the day of dose 2), 16 (0·1%) of 14 964 participants in the vaccine group and 62 (1·3%) of 4902 in the placebo group were confirmed to have COVID-19; vaccine efficacy was 91·6% (95% CI 85·6-95·2). Most reported adverse events were grade 1 (7485 [94·0%] of 7966 total events). 45 (0·3%) of 16 427 participants in the vaccine group and 23 (0·4%) of 5435 participants in the placebo group had serious adverse events; none were considered associated with vaccination, with confirmation from the independent data monitoring committee. Four deaths were reported during the study (three [<0·1%] of 16 427 participants in the vaccine group and one [<0·1%] of 5435 participants in the placebo group), none of which were considered related to the vaccine.
Interpretation: This interim analysis of the phase 3 trial of Gam-COVID-Vac showed 91·6% efficacy against COVID-19 and was well tolerated in a large cohort.
Funding: Moscow City Health Department, Russian Direct Investment Fund, and Sberbank.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Sputnik V COVID-19 vaccine candidate appears safe and effective.Lancet. 2021 Feb 20;397(10275):642-643. doi: 10.1016/S0140-6736(21)00191-4. Epub 2021 Feb 2. Lancet. 2021. PMID: 33545098 Free PMC article. No abstract available.
-
Countries split from EU on COVID-19 vaccines.Lancet. 2021 Mar 13;397(10278):958. doi: 10.1016/S0140-6736(21)00620-6. Lancet. 2021. PMID: 33714379 Free PMC article. No abstract available.
-
Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial - Authors' reply.Lancet. 2021 May 22;397(10288):1883-1884. doi: 10.1016/S0140-6736(21)00894-1. Epub 2021 May 12. Lancet. 2021. PMID: 33991474 Free PMC article. No abstract available.
-
Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial.Lancet. 2021 May 22;397(10288):1881-1883. doi: 10.1016/S0140-6736(21)00899-0. Epub 2021 May 12. Lancet. 2021. PMID: 33991475 Free PMC article. No abstract available.
References
-
- WHO Draft landscape of COVID-19 candidate vaccines. Jan 22, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

