Skin care interventions in infants for preventing eczema and food allergy
- PMID: 33545739
- PMCID: PMC8094581
- DOI: 10.1002/14651858.CD013534.pub2
Skin care interventions in infants for preventing eczema and food allergy
Update in
-
Skin care interventions in infants for preventing eczema and food allergy.Cochrane Database Syst Rev. 2022 Nov 14;11(11):CD013534. doi: 10.1002/14651858.CD013534.pub3. Cochrane Database Syst Rev. 2022. PMID: 36373988 Free PMC article.
Abstract
Background: Eczema and food allergy are common health conditions that usually begin in early childhood and often occur together in the same people. They can be associated with an impaired skin barrier in early infancy. It is unclear whether trying to prevent or reverse an impaired skin barrier soon after birth is effective in preventing eczema or food allergy.
Objectives: Primary objective To assess effects of skin care interventions, such as emollients, for primary prevention of eczema and food allergy in infants Secondary objective To identify features of study populations such as age, hereditary risk, and adherence to interventions that are associated with the greatest treatment benefit or harm for both eczema and food allergy.
Search methods: We searched the following databases up to July 2020: Cochrane Skin Specialised Register, CENTRAL, MEDLINE, and Embase. We searched two trials registers and checked reference lists of included studies and relevant systematic reviews for further references to relevant randomised controlled trials (RCTs). We contacted field experts to identify planned trials and to seek information about unpublished or incomplete trials.
Selection criteria: RCTs of skin care interventions that could potentially enhance skin barrier function, reduce dryness, or reduce subclinical inflammation in healthy term (> 37 weeks) infants (0 to 12 months) without pre-existing diagnosis of eczema, food allergy, or other skin condition were included. Comparison was standard care in the locality or no treatment. Types of skin care interventions included moisturisers/emollients; bathing products; advice regarding reducing soap exposure and bathing frequency; and use of water softeners. No minimum follow-up was required.
Data collection and analysis: This is a prospective individual participant data (IPD) meta-analysis. We used standard Cochrane methodological procedures, and primary analyses used the IPD dataset. Primary outcomes were cumulative incidence of eczema and cumulative incidence of immunoglobulin (Ig)E-mediated food allergy by one to three years, both measured by the closest available time point to two years. Secondary outcomes included adverse events during the intervention period; eczema severity (clinician-assessed); parent report of eczema severity; time to onset of eczema; parent report of immediate food allergy; and allergic sensitisation to food or inhalant allergen.
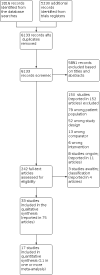
Main results: This review identified 33 RCTs, comprising 25,827 participants. A total of 17 studies, randomising 5823 participants, reported information on one or more outcomes specified in this review. Eleven studies randomising 5217 participants, with 10 of these studies providing IPD, were included in one or more meta-analysis (range 2 to 9 studies per individual meta-analysis). Most studies were conducted at children's hospitals. All interventions were compared against no skin care intervention or local standard care. Of the 17 studies that reported our outcomes, 13 assessed emollients. Twenty-five studies, including all those contributing data to meta-analyses, randomised newborns up to age three weeks to receive a skin care intervention or standard infant skin care. Eight of the 11 studies contributing to meta-analyses recruited infants at high risk of developing eczema or food allergy, although definition of high risk varied between studies. Durations of intervention and follow-up ranged from 24 hours to two years. We assessed most of this review's evidence as low certainty or had some concerns of risk of bias. A rating of some concerns was most often due to lack of blinding of outcome assessors or significant missing data, which could have impacted outcome measurement but was judged unlikely to have done so. Evidence for the primary food allergy outcome was rated as high risk of bias due to inclusion of only one trial where findings varied when different assumptions were made about missing data. Skin care interventions during infancy probably do not change risk of eczema by one to two years of age (risk ratio (RR) 1.03, 95% confidence interval (CI) 0.81 to 1.31; moderate-certainty evidence; 3075 participants, 7 trials) nor time to onset of eczema (hazard ratio 0.86, 95% CI 0.65 to 1.14; moderate-certainty evidence; 3349 participants, 9 trials). It is unclear whether skin care interventions during infancy change risk of IgE-mediated food allergy by one to two years of age (RR 2.53, 95% CI 0.99 to 6.47; 996 participants, 1 trial) or allergic sensitisation to a food allergen at age one to two years (RR 0.86, 95% CI 0.28 to 2.69; 1055 participants, 2 trials) due to very low-certainty evidence for these outcomes. Skin care interventions during infancy may slightly increase risk of parent report of immediate reaction to a common food allergen at two years (RR 1.27, 95% CI 1.00 to 1.61; low-certainty evidence; 1171 participants, 1 trial). However, this was only seen for cow's milk, and may be unreliable due to significant over-reporting of cow's milk allergy in infants. Skin care interventions during infancy probably increase risk of skin infection over the intervention period (RR 1.34, 95% CI 1.02 to 1.77; moderate-certainty evidence; 2728 participants, 6 trials) and may increase risk of infant slippage over the intervention period (RR 1.42, 95% CI 0.67 to 2.99; low-certainty evidence; 2538 participants, 4 trials) or stinging/allergic reactions to moisturisers (RR 2.24, 95% 0.67 to 7.43; low-certainty evidence; 343 participants, 4 trials), although confidence intervals for slippages and stinging/allergic reactions are wide and include the possibility of no effect or reduced risk. Preplanned subgroup analyses show that effects of interventions were not influenced by age, duration of intervention, hereditary risk, FLG mutation, or classification of intervention type for risk of developing eczema. We could not evaluate these effects on risk of food allergy. Evidence was insufficient to show whether adherence to interventions influenced the relationship between skin care interventions and risk of developing eczema or food allergy.
Authors' conclusions: Skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema, and probably increase risk of skin infection. Effects of skin care interventions on risk of food allergy are uncertain. Further work is needed to understand whether different approaches to infant skin care might promote or prevent eczema and to evaluate effects on food allergy based on robust outcome assessments.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
My institution received a grant from L'Oreal (La Roche Possay) for ‘An investigation of the skin barrier restoring effects of a cream containing ceramides in a multi vesicular emulsion in people with dry, eczema‐prone, skin (RESTORE)’ (clinical trial).
My institution received a grant from Johnson & Johnson for ‘A randomised controlled trial of a specially designed wash product and lotion for the maintenance of healthy skin and mind in babies (BOND)’.
My institution and I personally received payment for study design and steering, for travel to meetings for study or other purposes, and for participation in review activities (such as data monitoring boards, statistical analysis, end point committees) from Hyphens Pharma, L'Oreal (La Roche Possay), and Johnson & Johnson.
I received personal payment for consultancy, travel/accommodations/meeting expenses unrelated to activities pre‐mentioned, and lectures from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema; Pfizer with regard to biologic and topical drug trials for atopic eczema; and Galapagos and Kymab with regard to biologic drug trials for atopic eczema.
My institution received grants/grants pending from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema; Pfizer with regard to biologic and topical drug trials for atopic eczema; and Galapagos and Kymab with regard to biologic drug trials for atopic eczema.
My institution and I personally received payment for review preparation from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema; Pfizer with regard to biologic and topical drug trials for atopic eczema; and Galapagos and Kymab with regard to Biologic drug trials for atopic eczema.
My institution received payment for development of educational presentations from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema.
I am or have been an Investigator and Consultant for the following organisations: Astellas, Boots, Dermavant, Galapagos, Galderma, Hyphens, Johnson & Johnson, Kymab, LEO Pharma, L’Oreal, Menlo, Novartis, Oxagen, Pfizer, Procter & Gamble, Reckitt Benckiser, Regeneron, and Sanofi Genzyme.
Reports an NIHR RfPB grant awarded to Nottingham University Hospitals Trust. My employing institution, Imperial College London, has a formal research and innovation partnership with Nestle. This partnership does not directly involve me, and I do not think it is relevant to this review, but Nestle do have skin care products and products for prevention or treatment of allergic conditions within their portfolio.
Figures
































































References
References to studies included in this review
Abraham 2019 {published data only (unpublished sought but not used)}
-
- Abraham D, Ravindran V, Joseph M. Effectiveness of chlorhexidine bath, saline bath and standard bath on skin health status. Indian Journal of Public Health Research and Development 2019;10(8):563-568. [DOI: 10.37506/ijphrd.v10i8.7446] - DOI
Amer 2017 {published data only}
-
- Amer M, Amer A. Neonatal skin care. A four-week follow up randomized controlled trial. European Journal of Pediatric Dermatology 2015;25(2):93-99. [DOI: 10.26326/2281-9649.25.2.1106] - DOI
Baldwin 2001 {published data only}
-
- Baldwin S, Odio MR, Haines SL, O'Connor RJ, Englehart JS, Lane AT. Skin benefits from continuous topical administration of a zinc oxide/petrolatum formulation by a novel disposable diaper. Journal of the European Academy of Dermatology and Venereology: JEADV 2001;15(s1):5-11. [DOI: 10.1046/j.0926-9959.2001.00002.x] - DOI - PubMed
Bellemere 2018 {published data only}
-
- Bellemere G, Boyer G, De Belilovsky C, Baudouin C. Prevention of atopic dermatitis using emollients for 6 months-follow-up for 24 months. Pediatric Dermatology 2019;36:S9. [DOI: 10.1111/pde.13846] - DOI
-
- Bellemere G, Boyer G, De Belilovsky C, Moga A, Fontanie M, Baudouin C. Early atopic dermatitis: prevention biological data. Pediatric Dermatology 2017;34:S106-S107. [DOI: 10.1111/pde.13196] - DOI
-
- Bellemere G, Boyer G, De Belilovsky C, Moga A, Fontanie M, Baudouin C. Early atopic dermatitis: prevention study. Pediatric Dermatology 2018;35:S4‐S5. [DOI: 10.1111/pde.13502] - DOI
-
- Bellemere G, Boyer G, de Belilovsky C, Moga A, Fontaine M. Early atopic dermatitis: prevention biologic data. Journal of the American Academy of Dermatology 2018;79(3):AB115. [DOI: 10.1016/j.jaad.2018.05.487] - DOI
Chalmers 2020 {published and unpublished data}
-
- Chalmers JR, Haines RH, Mitchell EJ, Thomas KS, Brown SJ, Ridd M, et al. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (the BEEP trial): protocol for a randomised controlled trial. Trials 2017;18(1):343. [DOI: 10.1186/s13063-017-2031-3] - DOI - PMC - PubMed
-
- Chalmers JR, Thomas KS, Montgomery A, Sach T, Boyle RJ, Ridd MJ, et al. A protocol for a randomized controlled trial to determine whether application of emollient from birth can prevent eczema in high-risk children (BEEP Trial) (PP01). British Journal of Dermatology 2014;170(6):e32. [DOI: 10.1111/bjd.13064] - DOI
-
- ISRCTN21528841. Barrier enhancement for eczema prevention. www.isrctn.com/ISRCTN21528841 (first received 25 July 2014). [DOI: 10.1186/ISRCTN21528841] - DOI
Cooke 2015 {published and unpublished data}
-
- Cooke A, Cork M, Victor S, Campbell M, Danby S, Chittock J, et al. A pilot, assessor-blinded, randomized controlled trial of topical oils for neonatal skin. British Journal of Dermatology 2015;173:153. [DOI: 10.1111/bjd.13819] - DOI
-
- Cooke A, Cork MJ, Victor S, Campbell M, Danby S, Chittock J, et al. Olive oil, sunflower oil or no oil for baby dry skin or massage: a pilot, assessor-blinded, randomized controlled trial (the Oil in Baby SkincaRE [OBSeRvE] Study). Acta Dermato-Venereologica 2016;96(3):323-30. [DOI: 10.2340/00015555-2279] - DOI - PubMed
-
- ISRCTN37373893. The use of oil in baby skincare trial. www.isrctn.com/ISRCTN37373893 (first received 22 August 2013). [DOI: 10.1186/ISRCTN37373893] - DOI
Da Cunha 2008 {published data only}
-
- Da Cunha M, Procianoy R, Franceschini D, De Oliveira L, Cunha ML. Effect of the first bath with chlorhexidine on skin colonization with Staphylococcus aureus in normal healthy term newborns. Scandinavian Journal of Infectious Diseases 2008;40(8):615-20. [DOI: 10.1080/00365540801932447] - DOI - PubMed
Dissanayake 2019 {published and unpublished data}
-
- Dissanayake E, Tani Y, Nagai K, Sahara M, Mitsuishi C, Togawa Y, et al. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial, randomized, non-treatment controlled trial. International Archives of Allergy & Immunology 2019;180(3):202-11. [DOI: 10.1159/000501636] - DOI - PubMed
-
- Dissanayake E, Tani Y, Sahara M, Mitsuishi C, Nagai K, Sato Y, et al. Skincare and synbiotics for the prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial randomized non-treatment controlled trial. Allergy: European Journal of Allergy and Clinical Immunology 2018;73(105):692-3. [DOI: 10.1111/all.13539] - DOI - PubMed
-
- JPRN-UMIN000010838. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial, randomized, non-treatment controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000010838 (first received 31 May 2013). - PubMed
Dizon 2010 {published data only}
Duan 2019 {published data only}
-
- NCT02981056. A clinical assessment of the efficacy and effects of different skincare regimens on infants. clinicaltrials.gov/ct2/show/NCT02981056 (first received 2 December 2016).
Garcia Bartels 2010 {published data only}
Garcia Bartels 2011 {published data only}
Garcia Bartels 2012 {published data only}
-
- Garcia Bartels N, Massoudy L, Scheufele R, Dietz E, Proquitté H, Wauer R, et al. Standardized diaper care regimen: a prospective, randomized pilot study on skin barrier function and epidermal IL-1α in newborns. Pediatric Dermatology 2012;29(3):270‐6. [DOI: 10.1111/j.1525-1470.2011.01590.x] - DOI - PubMed
-
- NCT01131403. Clinical analysis of the influence of using the skin care products on the diaper area in comparison with using a cotton wool cloth, moistened with clear water on the skin physiology of the newborns from the 1st day to the 4th week of life. clinicaltrials.gov/ct2/show/NCT01131403 (first received 27 May 2010).
Garcia Bartels 2014 {published data only}
Horimukai 2014 {published and unpublished data}
-
- JPRN-UMIN000004544. Effect of emollients on the prevention of infantile eczema and atopic dermatitis. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000004544 (first received 1 November 2010).
-
- Ohya Y, Morita K, Narita M, Futamura M, Kondo M, Kitazawa H, et al. Primary prevention of atopic dermatitis by skin care with emollient: a randomized controlled study (OP02). British Journal of Dermatology 2014;170(6):e9. [DOI: 10.1111/bjd.13064] - DOI
Kataoka 2010 {published data only}
-
- Kataoka Y, Yoshida N, Nishino H, Maeda N, Sarumaru T, Kijima A, et al. Can skin care from neonatal period prevent the onset of atopic dermatitis? Allergo Journal 2010;19:338-9. [DOI: 10.1007/s40629-019-00114-5] - DOI
Lavender 2011 {published data only}
-
- ISRCTN72285670. Baby Skin Care Trial: a study comparing an infant skin-cleansing product with water. www.isrctn.com/ISRCTN72285670 (first received 17 March 2009). [DOI: 10.1186/ISRCTN72285670] - DOI
Lavender 2012 {published data only}
-
- ISRCTN86207019. Baby skin care research programme: Baby Wipes study. www.isrctn.com/ISRCTN86207019 (first received 17 February 2010). [DOI: 10.1186/ISRCTN86207019] - DOI
-
- Lavender T, Furber C, Cork M, Campbell M, Victor S. Baby skin care research programme: assessor-blinded randomised controlled trial comparing impregnated cleansing wipes with water in infants. Archives of Disease in Childhood: Fetal and Neonatal Edition 2011;1:Fa43-4. [DOI: 10.1136/archdischild.2011.300164.91] - DOI
Lavender 2013 {published data only}
-
- Lavender T, Bedwell C, Roberts SA, Hart A, Turner MA, Carter LA, et al. Randomized, controlled trial evaluating a baby wash product on skin barrier function in healthy, term neonates. Journal of Obstetric, Gynaecologic, and Neonatal Nursing 2013;42(2):203‐14. [DOI: 10.1111/1552-6909.12015] - DOI - PMC - PubMed
Lowe 2018a {published and unpublished data}
-
- ACTRN12609000727246. Prevention of eczema by a barrier lipid equilibrium strategy (PEBBLES) pilot study - Testing the compliance and safety of a strategy for improving infant skin function. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000727246 (first received 19 August 2009).
-
- ACTRN12613000472774. The PEBBLES study: Prevention of Eczema By a Barrier Lipid Equilibrium Strategy. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364115 (first received 23 April 2013).
-
- ACTRN12617001380381. The PEBBLES study – testing a strategy for preventing eczema and food allergy in high risk infants. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373506 (first received 20 September 2017).
-
- Lowe A, Su J, Allen K Abramson M, Cranswick N, Robertson C, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the pebbles pilot study NCD. Pediatric Dermatology 2017;34(S1):S35-6. [DOI: 10.1111/pde.13195] - DOI - PubMed
Lund 2020 {published data only}
McClanahan 2019 {published and unpublished data}
-
- McClanahan D, Wong A, Kezic S, Samrao A, Hajar T, Hill E, et al. A randomized controlled trial of an emollient with ceramide and filaggrin-associated amino acids for the primary prevention of atopic dermatitis in high-risk infants. Journal of the European Academy of Dermatology & Venereology 2019;33(11):2807-94. [DOI: 10.1111/jdv.15786] - DOI - PubMed
-
- NCT01375205. Comparing Cetaphil Restoraderm System versus standard skin care in infants. clinicaltrials.gov/ct2/show/NCT01375205 (first received 17 June 2011).
Migacheva 2018 {published data only}
-
- Migacheva N, Zhestkov A. Combined approach to primary prevention of atopic dermatitis. Allergy 2018;73:331. [DOI: 10.1111/all.13537] - DOI
NCT03376243 {unpublished data only}
-
- NCT03376243. EARLYEMOLLIENT - Feasibility of early emollient use in children with atopic eczema. clinicaltrials.gov/ct2/show/NCT03376243 (first received 18 December 2017).
Raisi Dehkordi 2010 {published data only}
-
- IRCT201102265912N1. The effect of massage with sunflower oil and sesame oil. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201102265912N1 (first received 8 January 2012).
-
- Raisi Dehkordi Z. A comparative study of the effect of massage with sunflower oil or sesame oil on infants' crying and sleep times: a randomized control trial. European Psychiatry 2014;29(Suppl 1):1. [DOI: 10.1016/S0924-9338(14)78247-1] - DOI
Rush 1986 {published data only}
-
- Rush J. Does routine newborn bathing reduce Staphylococcus aureus colonization rates? A randomized controlled trial. Birth 1986;13(3):176‐80. - PubMed
Sankaranarayanan 2005 {published data only}
-
- Sankaranarayanan K, Mondkar JA, Chauhan MM, Mascarenhas BM, Mainkar AR, Salvi RY. Oil massage in neonates: an open randomized controlled study of coconut versus mineral oil. Indian Pediatrics 2005;42(9):877‐84. [PMID: ] - PubMed
Simpson 2014 {published and unpublished data}
-
- Chalmers JR, Simpson EL, Chen YY, Cork MJ, Brown SJ, Thomas KS, et al. Feasibility study of barrier enhancement for eczema prevention (BEEP). British Journal of Dermatology 2014;170:e8-e9. [DOI: 10.1186/ISRCTN84854178] - DOI
-
- Glatz M, Polley EC, Schmid-Grendelmeier P, Simpson EL, Kong HH. Emollient therapy alters barrier function and skin microbes in infants at risk for developing atopic dermatitis. Swiss Medical Weekly 2017;147(Suppl 228):53S.
Skjerven 2020 {published and unpublished data}
-
- Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Department of Error: Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial (The Lancet (2020) 395(10228) (951-61) (S0140673619329836)). Lancet 2020;395(10228):e53. [DOI: 10.1016/S0140-6736(20)30422-0] - DOI - PubMed
-
- Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2020;395(10228):951‐961. [DOI: 10.1016/S0140-6736(19)32983-6] - DOI - PubMed
Thitthiwong 2019 {published data only}
-
- Koopitakkajorn T, Thitthiwong P. The good skin care practices and emollient using since early infancy as the primary prevention of infantile atopic dermatitis in infants at risk: a randomized controlled trial. Pediatric Dermatology 2017;34(S1):S35. [DOI: 10.1111/pde.13195] - DOI
-
- TCTR20161208001. The good skin care practices and emollient using since early infancy as the primary prevention of infantile atopic dermatitis in infants at risk: a randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20161208001 (first received 4 December 2016).
Tielsch 2007 {published data only}
-
- NCT00109616. Community trial of newborn skin and umbilical cord cleansing on neonatal mortality in Nepal. clinicaltrials.gov/ct2/show/NCT00109616 (first received 2 May 2005).
Yonezawa 2018 {published and unpublished data}
-
- JPRN-UMIN000013260. Effects of moisturizing skin care from the neonatal stage for improving skin barrier function and preventing skin trouble. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000013260 (first received 1 March 2014).
References to studies excluded from this review
ACTRN12607000466448 {published data only}
-
- ACTRN12607000466448. An intervention to reduce the prevalence and impact of asthma and food allergies occurring in association with atopic dermatitis through improved skin care in infants and young children. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82278 (first received 11 September 2007).
Ahmed 2007 {published data only}
Alonso 2013 {published data only}
-
- ISRCTN00356649. Efficacy of petroleum jelly in the prevention of irritant diaper dermatitis. www.isrctn.com/ISRCTN00356649 (first received 8 October 2010).
Baer 2006 {published data only}
Barria 2004 {published data only}
-
- Barría K, Muñoz C, Pérez‐Cotapos ML, Fehlmann E, Brockmann P, Tapia JL. Comparison of 2 models for the initial skin care in the healthy full term newborn infant. Pediatric Dermatology 2004;21:317-18. [DOI: 10.1111/j.0736-8046.2004.21332.x] - DOI
Baudouin 2014 {published data only}
-
- Baudouin C, De Belilovsky C, Lachmann N, Bredif S, Menu F, Msika P. Tolerance assessment of baby's skin products: Innovative approaches using stinging test and infant epidermis model. Journal of Investigative Dermatology 2014;2:S51.
Baudouin 2014a {published data only}
-
- Baudouin C, De Belilovsky C, Menu F, Lachmann N, Msika P, Bredif S. Tolerance assessment of cosmetic products dedicated to baby's skin: innovative approaches using stinging test and infant epidermis model. Journal of the American Academy of Dermatology 2014;1:AB149. [DOI: 10.1016/j.jaad.2014.01.619] - DOI
Berger 2009 {published data only}
-
- Berger C, Inzinger R. Study of skin care in premature and newborn infants. Kinderkrankenschwester 2009;28(3):116-25. - PubMed
Bhakoo 1969 {published data only}
-
- Bhakoo ON, Lall JC, Agarwal KC. Prevention of hospital infections in neonates: an evaluation of no bath regimen. Indian Pediatrics 1969;6(1):697‐700. - PubMed
Blume Peytavi 2010 {published data only}
-
- Blume-Peytavi U, Garcia Bartels N. Neonatal skin care influence of standardized skin care regimen on skin barrier function. Aktuelle Dermatologie 2010;36(6):214-6.
Blume Peytavi 2012 {published data only}
-
- Blume-Peytavi U, Hauser M, Stamatas GN, Pathirana D, Garcia Bartels N. Skin care practices for newborns and infants: Review of the clinical evidence for best practices. Pediatric Dermatology 2012;29:1-14. - PubMed
Blume Peytavi 2014 {published data only}
-
- Blume-Peytavi U, Hauser M, Lunnemann L, Stamatas GN, Kottner J, Garcia Bartels N. Prevention of diaper dermatitis in infants--a literature review. Pediatric Dermatology 2014;31(4):413-29. - PubMed
Blume Peytavi 2016 {published data only}
-
- Blume-Peytavi U. Improved understanding of infant skin physiology, maturation and skin care. European Journal of Pediatric Dermatology 2016;26(3):166.
Brandon 2010 {published data only}
Bryanton 2004 {published data only}
Chaithirayanon 2016 {published data only}
-
- Chaithirayanon S. Comparative study between talcum and zinc oxide cream for the prevention of irritant contact diaper dermatitis in infants. Journal of the Medical Association of Thailand 2016;99(Suppl 8):S1‐S6. [PMID: ] - PubMed
Chen 2009 {published data only}
-
- Chen HY. The clinic effect of Chinese medicine bath and massage on pathological jaundice of newborn. China Foreign Medical Treatment 2009;28(18):128.
Cleminson 2015 {published data only}
-
- Cleminson J, McGuire W. Topical emollient for prevention of infection in preterm infants: a systematic review. Lancet 2015;385:S31. [PMID: ] - PubMed
Cleminson 2016 {published data only}
Conner 2004 {published data only}
Cooke 2014 {published data only}
-
- Cooke A, Victor S, Cork M, Lavender T. Topical oils for the prevention or treatment of dry skin in term infants. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD011100. [DOI: 10.1002/14651858.CD011100] - DOI
Cooke 2018 {published data only}
-
- Cooke A, Bedwell C, Campbell M, McGowan L, Ersser SJ, Lavender T. Skin care for healthy babies at term: a systematic review of the evidence. Midwifery 2018;56:29-43. [PMID: ] - PubMed
Cowan 1986 {published data only}
CTRI201208002876 {unpublished data only}
-
- CTRI/2012/08/002876. A prospective, open label clinical trial to evaluate the efficacy and safety of Tubby Nutririch lotion for the treatment of dry skin with or without atopic tendency in infants and children. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5054 (first received 9 August 2012).
CTRI201709009890 {published data only}
-
- CTRI/2017/09/009890. Comparing the effect of three different skin antiseptic preparations in neonates (first 28 days of life) - randomized trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19272 (first received 22 September 2017).
Da Cunha 2005 {published data only}
Da Cunha 2005a {published data only}
-
- Da Cunha ML, Procianoy RS. Skin microflora and bathing of preterm newborn infants. In: Pediatric Academic Societies Annual Meeting. Washington, DC, 2005.
Darmstadt 2004 {published data only}
-
- Darmstadt GL, Badrawi N, Law PA, Ahmed S, Bashir M, Iskander I, et al. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatric Infectious Disease Journal 2004;23(8):719-25. [DOI: 10.1097/01.inf.0000133047.50836.6f] - DOI - PubMed
Darmstadt 2005 {published data only}
-
- Darmstadt GL, Saha SK, Nawshad Uddin Ahmed ASM, Azad Chowdhury MAK, Law PA, Ahmed S, et al. Skin barrier-enhancing emollients prevent nosocomial infections in preterm infants in Bangladesh. In: Pediatric Academic Societies Annual Meeting. Washington DC, 2005.
Darmstadt 2005a {published data only}
-
- Darmstadt GL, Saha SK, Ahmed AS, Chowdhury MA, Law PA, Ahmed S, et al. Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet 2005;365(9464):1039‐45. [DOI: 10.1016/S0140-6736(05)71140-5] - DOI - PubMed
Darmstadt 2007 {published data only}
Darmstadt 2008 {published data only}
Darmstadt 2014 {published data only}
Duggan 2015 {published data only}
-
- Duggan C. Eczema on trial. Journal of Family Health 2015;25(3):10-12. [PMID: ] - PubMed
Erdemir 2015 {published data only}
Ernest 1995 {published data only}
EUCTR2005‐001269‐32‐AT {published data only}
-
- EUCTR2005-001269-32-AT. Clinical trial on skin care in neonatology. A comparison between two skin care products and no skin care. www.clinicaltrialsregister.eu/ctr-search/search?query=2005-001269-32 (first received 22 November 2005).
Fernandez 2018 {published data only}
Field 2008 {published data only}
-
- Field T, Field T, Cullen C, Largie S, Diego M, Schanberg S, et al. Lavender bath oil reduces stress and crying and enhances sleep in very young infants. Early Human Development 2008;84(6):399‐401. - PubMed
Fluhr 2012 {published data only}
Foisy 2011 {published data only}
Franck 2000 {published data only}
-
- Franck LS, Quinn D, Zahr L. Effect of less frequent bathing of preterm infants on skin flora and pathogen colonization. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2000;29(6):584‐9. - PubMed
Friscia 2019 {published data only (unpublished sought but not used)}
Gao 2008 {published data only}
-
- Gao Y. Effectiveness observation on moisturizer for infant eczema. Chinese Journal of Leprosy and Skin Disease 2008;24(4):294.
Garcia Bartels 2009 {published data only}
-
- Garcia Bartels N, Mleczko A, Schink T, Proquitte H, Wauer RR, Blume-Peytavi U. Influence of bathing or washing on skin barrier function in newborns during the first four weeks of life. Skin Pharmacology & Physiology 2009;22(5):248-57. - PubMed
Gezon 1964 {published data only}
-
- Gezon HM, Thompson DJ, Rogers KD, Hatch TF, Taylor PM. Hexachlorophene bathing in early infancy. New England Journal of Medicine 1964;270:379‐86. - PubMed
Gfatter 1997 {published data only}
Gleiss 1965 {published data only}
-
- Gleiss J, Sommerkamp B. Comparative study of soapless skin cleansing of infants. Archiv fur Kinderheilkunde 1965;172(2):154-60. - PubMed
Gunt 2018 {published data only}
-
- Gunt HB, Levy SB, Lutrario CA. A natural cream-to-powder formulation developed for the prevention of diaper dermatitis in diaper-wearing infants and children: barrier property and in-use tolerance studies. Journal of Drugs in Dermatology 2018;17(5):566‐70. - PubMed
Hauk 2008 {published data only}
-
- Hauk PJ. The role of food allergy in atopic dermatitis. Current Allergy and Asthma Reports 2008;8(3):188-94. - PubMed
Hawkins 2017 {published data only}
-
- Hawkins S, Edmunds M, Foy V, Vincent C, Cox E, O'Shea S, et al. Superior skin care for infants with a new nourishing regimen containing stearic acid, petrolatum, and fatty acids: tip-to-toe-wash and lotion. Pediatric Dermatology 2017;34(S1):S34. [DOI: 10.1111/pde.13195] - DOI
Hnatko 1977 {published data only}
Horimukai 2016 {published data only}
-
- Horimukai K, Morita K, Narita M, Kondo M, Kabashima S, Inoue E, et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergology International 2016;65(1):103-8. - PubMed
Hu 2014 {published data only}
-
- Hu X, Zhang Y. Effect of topically applied sunflower seed oil in preterm infants. Pediatric Critical Care Medicine 2014;15(4 Suppl 1):144‐5. [DOI: 10.1097/01.pcc.0000449366.67893.84] - DOI
IRCT201306164617N {published data only}
-
- IRCT201306164617N. Effect of bath on clinical outcomes in preterm infants: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201306164617N7 (first received 28 June 2013).
IRCT201306164617N7 {published data only}
-
- IRCT201306164617N7. Effect of bath on clinical outcomes in preterm infants: a randomized controlled trial. en.irct.ir/trial/4950 (first received 28 June 2013).
IRCT2013090814594N1 {published data only}
-
- IRCT2013090814594N1. A comparison between the effects of swaddled bathing and conventional bathing on physiological and behavioral parameters among the preterm neonates. en.irct.ir/trial/14191 (first received 3 January 2014).
IRCT2016111530903N {published data only}
-
- IRCT2016111530903N. The efficacy of Chamomile oil on infantile colic. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016111530903N1 (first received 4 April 2017).
IRCT20170911036118N1 {published data only}
-
- IRCT20170911036118N1. Effect of massage and washing in physiological jaundice. en.irct.ir/trial/27093 (first received 17 January 2018).
ISRCTN71423189 {published data only}
-
- ISRCTN71423189. Multi-centre randomised controlled trial of ion-exchange water softeners for the treatment of atopic eczema in children (Softened Water Eczema Trial). www.isrctn.com/ISRCTN71423189 (first received 8 January 2007). [DOI: 10.1186/ISRCTN71423189] - DOI
ISRCTN89579779 {published data only}
-
- ISRCTN89579779. The use of Aquaphor to prevent transepidermal water loss and to maintain electrolyte balance in preterm newborn infants in the first week of life. www.isrctn.com/ISRCTN89579779 (first received 30 September 2004). [DOI: 10.1186/ISRCTN89579779] - DOI
Jabraeile 2016 {published data only}
Jensen 1971 {published data only}
-
- Jensen JP. Transcutaneous absorption of boron from a baby ointment used prophylactically against diaper dermatitis. Nordisk Medicin 1971;86(48):1425‐9. - PubMed
JPRN UMIN000005158 {published data only}
-
- JPRN-UMIN000005158. Randomized controlled trial to evaluate clinical efficacy of once- or twice-daily skincare with emollient for prevention of atopic dermatitis exacerbation. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000005803 (first received 3 July 2011).
JPRN‐UMIN000018110 {published data only}
-
- JPRN-UMIN000018110. Preliminary study on the skin cleaning method without cleaning agent during bathing in the NICU - Comparison with the case of using a skin cleaning agent. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000018110 (first received 26 June 2015).
JPRN‐UMIN000025302 {published data only}
-
- JPRN-UMIN000025302. Open test of skin care products for newborns. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000025302 (first received 16 November 2017).
JPRN UMIN000032181 {published data only}
-
- JPRN-UMIN000032181. Study of neonates on the intestinal bacterial flora and skin conditions. upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000036709 (first received 10 April 2018).
JPRN UMIN000032798 {published data only}
-
- JPRN-UMIN000032798. Baby diaper study (No.2016-001). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000037416 (first received 31 May 2018).
JPRN UMIN000035357 {published data only}
-
- JPRN-UMIN000035357. Effect of moisturizer application on perianal area in neonates; comparison of effects on erythema and skin barrier function at 1-month checkup. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000040287 (first received 25 December 2018).
JPRN UMIN000035412 {published data only}
-
- JPRN-UMIN000035412. Effect of change in bathing and skin care in infancy on the incidence of eczema at 6 months of age. rctportal.niph.go.jp/en/detail?trial_id=UMIN000035412 (first received 7 January 2019).
Kadar 1974 {published data only}
-
- Kadar V, Osbourn R. The mildness of soaps in the care of infants' skin: a comparative study. Current Therapeutic Research - Clinical and Experimental 1974;16(5):452-6. [PMID: ] - PubMed
Kanti 2014 {published data only}
-
- Kanti V, Grande C, Stroux A, Buhrer C, Blume-Peytavi U, Garcia Bartels N. Influence of sunflower seed oil on the skin barrier function of preterm infants: a randomized controlled trial. Dermatology 2014;229(3):230-9. - PubMed
Kanti 2017 {published data only}
-
- Kanti V, Gunther M, Stroux A, Sawatzky S, Henrich W, Abou-Dakn M, et al. Influence of sunflower seed oil or baby lotion on the skin barrier function of newborns: a pilot study. Journal of Cosmetic Dermatology 2017;16(4):500-7. - PubMed
Kiechl Kohlendorfer 2008 {published data only}
Konar 2019 {published data only}
Koplin 2019 {published data only}
-
- Koplin JJ, Allen KJ, Tang MLK. Important risk factors for the development of food allergy and potential options for prevention. Expert Review of Clinical Immunology 2019;15(2):147-52. - PubMed
Kottner 2017 {published data only}
-
- Kottner J, Kanti V, Dobos G, Hahnel E, Lichterfeld-Kottner A, Richter C, et al. The effectiveness of using a bath oil to reduce signs of dry skin: a randomized controlled pragmatic study. International Journal of Nursing Studies 2017;65:17-24. - PubMed
Kvenshagen 2014 {published data only}
-
- Kvenshagen BK, Mowinckel P, Carlsen KH, Carlsen KL. Intensive oil baths from six weeks of age reduce xerosis and possibly atopic eczema in infancy. European Respiratory Journal 2012;40(Suppl 56):424s.
Lane 1993 {published data only}
-
- Lane AT, Drost SS. Effects of repeated application of emollient cream to premature neonates' skin. Pediatrics 1993;92(3):415‐9. - PubMed
Larson 2005 {published data only}
-
- Larson AA, Dinulos JG. Cutaneous bacterial infections in the newborn. Current Opinion in Pediatrics 2005;17(4):481-5. - PubMed
Lee 2018 {published data only}
LeFevre 2010 {published data only}
Leung 2015 {published data only}
-
- Leung DYM. Prophylactic emollient use beginning at birth prevents atopic dermatitis. Journal of Pediatrics 2015;166(3):777-8. - PubMed
Li 2016 {published data only}
-
- Li X, Zhong Q, Tang L. A meta-analysis of the efficacy and safety of using oil massage to promote infant growth. Journal of Pediatric Nursing 2016;31(5):e313-22. - PubMed
Ling 2011 {published data only}
-
- Ling L, Bi XY. Swimming and bathing with Chinese herbal medicine in treatment of neonatal jaundice: clinical analysis of 50 cases. Journal of Qilu Nursing 2011;17(1):42‐3.
Linnamaa 2010 {published data only}
-
- Linnamaa P, Savolainen J, Koulu L, Tuomasjukka S, Kallio H, Yang B, et al. Blackcurrant seed oil for prevention of atopic dermatitis in newborns: a randomized, double-blind, placebo-controlled trial. Clinical and Experimental Allergy 2010;40(8):1247‐55. [DOI: 10.1111/j.1365-2222.2010.03540.x] - DOI - PubMed
Lowe 2012 {published data only}
Lowe 2018b {published data only}
-
- Lowe AJ, Leung DYM, Tang MLK, Su JC, Allen KJ. The skin as a target for prevention of the atopic march. Annals of Allergy, Asthma, & Immunology 2018;120(2):145-51. - PubMed
Lund 2001 {published data only}
-
- Lund CH, Kuller J, Lane AT, Lott JW, Raines DA, Thomas KK. Neonatal skin care: evaluation of the AWHONN/NANN research-based practice project on knowledge and skin care practices. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2001;30(1):30‐40. - PubMed
Lund 2001a {published data only}
-
- Lund CH, Osborne JW, Kuller J, Lane AT, Lott JW, Raines DA. Neonatal skin care: clinical outcomes of the AWHONN/NANN evidence-based clinical practice guideline. Association of Women's Health, Obstetric and Neonatal Nurses and the National Association of Neonatal Nurses. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2001;30(1):41‐51. - PubMed
Marenholz 2015 {published data only}
-
- Marenholz I, Esparza-Gordillo J, Lee YA. The genetics of the skin barrier in eczema and other allergic disorders. Current Opinion in Allergy & Clinical Immunology 2015;15(5):426-34. - PubMed
Muggli 2009 {published data only}
-
- Muggli R. Natural management of napkin rash. European Journal of Pediatric Dermatology 2009;19(1):43‐6.
Nangia 2015 {published data only}
Natsume 2018 {published data only}
-
- Natsume O, Ohya Y. Recent advancement to prevent the development of allergy and allergic diseases and therapeutic strategy in the perspective of barrier dysfunction. Allergology International 2018;67(1):24-31. - PubMed
NCT00162747 {published data only}
-
- NCT00162747. Topical therapy for prevention of infections in preterm infants. clinicaltrials.gov/ct2/show/NCT00162747 (first received 13 September 2005).
NCT00257569 {published data only}
-
- NCT00257569. Study of atopic dermatitis in pediatrics. clinicaltrials.gov/ct2/show/NCT00257569 (first received 23 November 2005).
NCT00806221 {published data only}
-
- NCT00806221. An open-label study investigating the effects of early skin barrier protection on the development of atopic dermatitis. clinicaltrials.gov/ct2/show/NCT00806221 (first received 10 December 2008).
NCT00917085 {published data only}
-
- NCT00917085. Is skin-to-skin care helpful for preterm infants and their mothers after birth? clinicaltrials.gov/ct2/show/NCT00917085 (first received 10 June 2009).
NCT01131403 {published data only}
-
- NCT01131403. Clinical analysis of the influence of using the skin care products on the diaper area in comparison with using a cotton wool cloth, moistened with clear water on the skin physiology of the newborns from the 1st day to the 4th week of life. https://clinicaltrials.gov/ct2/show/NCT01131403 (first received 27 May 2010).
NCT01177111 {published data only}
-
- NCT01177111. Impact of sunflower seed oil massage on neonatal mortality and morbidity in Nepal. clinicaltrials.gov/show/NCT01177111 (first received 6 August 2010).
NCT01364948 {published data only}
-
- NCT01364948. Effect of coconut oil application in reducing water loss from skin of premature babies in first week of life (TEWL) (TopOilTewl). clinicaltrials.gov/ct2/show/NCT01364948 (first received 3 June 2011).
NCT01396642 {published data only}
-
- NCT01396642. Topical emollient therapy. clinicaltrials.gov/ct2/show/NCT01396642 (first received 19 July 2011).
NCT01758068 {published data only}
-
- NCT01758068. Effect of twice daily application of coconut oil in reducing water loss from skin of premature babies in first week of life. clinicaltrials.gov/ct2/show/NCT01758068 (first received 31 December 2012).
NCT02120833 {published data only}
-
- NCT02120833. A test to determine the usefulness and safety of a cream used on babies with dry itchy skin. www.clinicaltrials.gov/ct2/show/NCT02120833 (first received 7 September 2015).
NCT02403999 {published data only}
-
- NCT02403999. A tolerability assessment study of three wash products in infants. clinicaltrials.gov/ct2/show/NCT02403999 (first received 31 March 2015).
NCT02404493 {published data only}
-
- NCT02404493. Test to determine the effectiveness of moisturizing balm used on babies with dry, itchy skin. clinicaltrials.gov/ct2/show/NCT02404493 (first received 31 March 2015).
NCT02557698 {published data only}
-
- NCT02557698. Effectiveness of using an oil bath additive. clinicaltrials.gov/ct2/show/NCT02557698 (first received 23 September 2015).
NCT02614248 {published data only}
-
- NCT02614248. The use of coconut oil for the prevention and treatment of diaper dermatitis in the NICU population. clinicaltrials.gov/ct2/show/NCT02614248 (first received 25 November 2015).
NCT02857062 {published data only}
-
- To evaluate the in use tolerance of E45 eczema repair emollient in babies and children with (very(dry/atopic skin. clinicaltrials.gov/ct2/show/NCT02857062 (first received 5 August 2016).
NCT03089476 {published data only}
-
- NCT03089476. Evaluating skin barrier dysfunction in infants at high risk of atopy. clinicaltrials.gov/ct2/show/NCT03089476 (first received 24 March 2017).
NCT03112876 {published data only}
-
- NCT03112876. Sebum collection and skin barrier function analysis (Sebum). clinicaltrials.gov/ct2/show/NCT03112876 (first received 13 April 2017).
NCT03143504 {published data only}
-
- NCT03143504. A longitudinal investigation of skin barrier development from birth and the validation of early predictors of atopic eczema risk: the Skin Testing for Atopic eczema Risk (STAR) Study (STAR). clinicaltrials.gov/ct2/show/NCT03143504 (first received 8 May 2017).
NCT03719742 {published data only}
-
- NCT03719742. A clinical study to evaluate the safety and efficacy of a baby cleanser and a moisturizer. clinicaltrials.gov/ct2/show/NCT03719742 (first received 25 October 2018).
NCT03738163 {published data only}
-
- NCT03738163. A clinical investigation with Epaderm® cream (PD-539878). clinicaltrials.gov/ct2/show/NCT03738163 (first received 13 November 2018).
NCT03742414 {published data only}
-
- NCT03742414. Seal, stopping atopic dermatitis and allergy study. clinicaltrials.gov/ct2/show/NCT03742414 (first received 15 November 2018).
NCT03813472 {published data only}
-
- NCT03813472. Hydration sensor for atopic dermatitis. clinicaltrials.gov/ct2/show/results/NCT03813472 (first received 23 January 2019).
NCT04001855 {published data only}
-
- NCT04001855. Evaluating the effect of bathing additives in atopic dermatitis. clinicaltrials.gov/ct2/show/NCT04001855 (first received 28 June 2019).
NCT04099602 {published data only}
-
- NCT04099602. The effect of massage on bilirubin level in infants. clinicaltrials.gov/ct2/show/NCT04099602 (first received 23 September 2019).
Nesmiyanov 2018 {published data only}
-
- Nesmiyanov P, Gutov M, Strygin A, Tolkachev B, Morkovin E, Dotsenko A. M. vaccae-based formulation for the primary prevention of atopic dermatitis. Allergy 2018;73:107. [DOI: 10.1111/all.13535] - DOI
Nopper 1996 {published data only}
Noviello 2005 {published data only}
-
- Noviello MR. Effects after daily use of washing products on infants aged 0-52 weeks. Minerva Pediatrica 2005;57(6):411‐8. - PubMed
Pupala 2017 {published data only}
-
- Pupala S, Rao S, Strunk T, Patole S. Topical application of coconut oil to the skin of preterm infants-a systematic review. Journal of Paediatrics and Child Health 2017;53 Suppl 2:82. - PubMed
Pupala 2018 {published data only}
-
- Pupala S, Strunk T, Patole S, Doherty D, Hibbert J. Topical coconut oil to improve skin condition in very preterm infants: a pilot randomised clinical trial. Journal of Paediatrics and Child Health 2018;54:103‐4. [DOI: 10.1111/jpc.13882_280] - DOI
Pupala 2019 {published data only}
-
- Pupala SS, Rao S, Strunk T, Patole S. Topical application of coconut oil to the skin of preterm infants: a systematic review. European Journal of Pediatrics 2019;178(9):1317-24. - PubMed
Qiu 2008 {published data only}
-
- Qiu HM, Zhou L. The clinic effect of Chinese medicine bath therapy on 132 neonates with hyperbilirubinemia. Shaanxi Journal of Traditional Chinese Medicine 2008;29(11):1479‐80.
Quinn 2005 {published data only}
RBR 93996y {published data only}
-
- RBR-93996y. Effect of liquid and bar soap in healthy term newborns. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-93996y (first received 13 July 2018).
Rehbinder 2018 {published data only}
-
- Rehbinder EM, Winger AJ, Landro L, Carlsen KH, Carlsen MH, Fatnes TA, et al. Is dry skin in infants 3 months of age associated with increased transepidermal water loss? British Journal of Dermatology 2018;179(1):e59. [DOI: 10.1111/bjd.16718] - DOI
Rosenstock 2007 {published data only}
-
- Rosenstock. The impact of emollient therapy during the neonatal period on neurodevelopmental outcomes. Pediatric Academic Society 2007;8315:no pagination.
Sach 2019 {published data only}
Salam 2013 {published data only}
Salam 2015 {published data only}
Sarkar 2010 {published data only}
-
- Sarkar R, Basu S, Agrawal RK, Gupta P. Skin care for the newborn. Indian Pediatrics 2010;47(7):593-8. - PubMed
Sawatzky 2016 {published data only}
-
- Sawatzky S, Schario M, Stroux A, Lunnemann L, Zuberbier T, Blume-Peytavi U, et al. Children with dry skin and atopic predisposition: outcome measurement with validated scores for atopic dermatitis. Skin Pharmacology & Physiology 2016;29(3):148-56. - PubMed
Solanki 2005 {published data only}
-
- Solanki K, Matnani M, Kale M, Joshi K, Bavdekar A, Bhave S, et al. Transcutaneous absorption of topically massaged oil in neonates. Indian Pediatrics 2005;42(10):998‐1005. - PubMed
Soll 2000 {published data only}
Soriano 2000 {published data only}
-
- Soriano CR, Martinez FE, Jorge SM. Cutaneous application of vegetable oil as a coadjutant in the nutritional management of preterm infants. Journal of Pediatric Gastroenterology and Nutrition 2000;31(4):387-90. - PubMed
Summers 2017 {published data only}
-
- Summers A, Visscher M, Khatry S, Sherchand J, LeClerq S, Katz J, et al. Neonatal skin integrity: impact of mustard seed versus sunflower oil massage among newborns in rural Nepal. Pediatric Dermatology 2017;34:S85. [DOI: 10.1111/pde.13195] - DOI
TCTR20161209001 {published data only}
-
- TCTR20161209001. Efficacy of breast milk application on skin condition in preterm infants: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20161209001 (first received 9 December 2016).
Thomas 1979 {published data only}
Vaivre Douret 2009 {published data only}
-
- Vaivre-Douret L, Oriot D, Blossier P, Py A, Kasolter-Pere M, Zwang J. The effect of multimodal stimulation and cutaneous application of vegetable oils on neonatal development in preterm infants: a randomized controlled trial. Child: Care, Health & Development 2009;35(1):96-105. - PubMed
Visscher 2009 {published data only}
-
- Visscher M, Odio M, Taylor T, White T, Sargent S, Sluder L, et al. Skin care in the NICU patient: effects of wipes versus cloth and water on stratum corneum integrity. Neonatology 2009;96(4):226-34. - PubMed
Wananukul 2001 {published data only}
-
- Wananukul S, Praisuwanna P, Kesorncam K. Effects of clear topical ointment on transepidermal water loss in jaundiced preterm infants receiving phototherapy. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2001;84(6):837‐41. - PubMed
Wananukul 2002 {published data only}
-
- Wananukul S, Praisuwanna P. Clear topical ointment decreases transepidermal water loss in jaundiced preterm infants receiving phototherapy. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2002;85(1):102‐6. - PubMed
Wang 2009 {published data only}
-
- Wang Y L, Tan P, Wu SY, Gao GE, Chen B. The effect of Chinese medicine bath therapy on the levels of neonatal bilirubin. China Pharmacist 2009;12(11):1622‐3.
Waserman 2016 {published data only}
-
- Waserman S. Doctor, can we prevent food allergy and eczema in our baby? Current Opinion in Allergy & Clinical Immunology 2016;16(3):265-71. - PubMed
Xatzopoulou 2010 {published data only}
-
- Xatzopoulou S, Liosis G, Giannouli O, Grigoriadou D, Kyriacou M. Olive oil as a potential prebiotic in infants' skin. Early Human Development 2010;1:S20.
Xiao 2009 {published data only}
-
- Xiao DM, Wu CL, Liu QL. Traditional Chinese medicine bath with the sun on the prevention of neonatal jaundice. Chinese Medicine Modern Distance Education of China 2009;7(3):19.
Yamamoto 1996 {published data only}
-
- Yamamoto K. Soaps and detergents in children. Clinics in Dermatology 1996;14(1):81-4. - PubMed
Zhang 2016 {published data only}
-
- Zhang SZ, Xu ZH, Zhao LH. Observation on the effect of Shule liniment for neonatal touch combined with acupoint massage in the treatment of neonatal rash. Guangming Journal of Chinese Medicine [Guang Ming Zhong Yi Za Zhi] 2016;31(1):63‐4.
References to studies awaiting assessment
ISRCTN38965585 {published data only}
-
- CTRI/2014/12/005282. Can an intervention involving improvements in community-based newborn massage practice with promotion of cold-pressed sunflower oil as preferred emollient improve newborn survival in rural North India? www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/12/005282 (first received 11 December 2014).
-
- ISRCTN38965585. Can an intervention involving improvements in community-based newborn massage practice with promotion of cold-pressed sunflower oil as preferred emollient improve newborn survival in rural North India? www.isrctn.com/ISRCTN38965585 (first received 8 July 2014). [DOI: 10.1186/ISRCTN38965585] - DOI
JPRN‐UMIN000026877 {published data only}
-
- JPRN-UMIN000026877. Use test of skin care goods. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000026877 (first received 5 April 2017).
NCT03640897 {published data only}
-
- NCT03640897. Evaluation of the safety and performance of LiNiDERM® in the prevention of infant diaper rash. clinicaltrials.gov/ct2/show/NCT03640897 (first received 21 August 2018).
References to ongoing studies
Eichner 2020 {published data only}
-
- Eichner B, Michaels LAC, Branca K, Ramsey K, Mitchell J, Morris CD, et al. A Community-based Assessment of Skin Care, Allergies, and Eczema (CASCADE): an atopic dermatitis primary prevention study using emollients-protocol for a randomized controlled trial. Trials 2020;21(1):243. [DOI: 10.1186/s13063-020-4150-5] - DOI - PMC - PubMed
-
- NCT03409367. A Community-based Assessment of Skin Care, Allergies, and Eczema (CASCADE). clinicaltrials.gov/ct2/show/NCT03409367 (first received 24 January 2018).
Jabbar‐Lopez 2019 {published data only}
-
- Jabbar-Lopez ZK, Gurung N, Greenblatt D, Briley A, Chalmers JR, Thomas KS, et al. Protocol for an outcome assessor-blinded pilot randomised controlled trial of an ion-exchange water softener for the prevention of atopic eczema in neonates, with an embedded mechanistic study: the Softened Water for Eczema Prevention (SOFTER) trial. BMJ Open 2019;9(8):e027168. [DOI: 10.1136/bmjopen-2018-027168] - DOI - PMC - PubMed
-
- NCT03270566. Softened water for eczema prevention pilot trial. clinicaltrials.gov/ct2/show/NCT03270566 (first received 1 September 2017).
Lowe 2019 {published data only}
-
- ACTRN12617001380381. The PEBBLES Study - testing a strategy for preventing eczema and food allergy in high risk infants. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001380381 (first received 28 September 2017).
-
- Lowe A, Su J, Tang M, Lodge CJ, Matheson M, Allen KJ, et al. PEBBLES study protocol: a randomised controlled trial to prevent atopic dermatitis, food allergy and sensitisation in infants with a family history of allergic disease using a skin barrier improvement strategy. BMJ Open 2019;9(3):e024594. [DOI: 10.1136/bmjopen-2018-024594] - DOI - PMC - PubMed
NCT02906475 {published data only}
-
- NCT02906475. Atopic dermatitis in atopy predisposed infants. clinicaltrials.gov/ct2/show/NCT02906475 (first received 20 September 2016).
NCT03142984 {published data only}
-
- NCT03142984. UK BABY study using a baby wash and lotion regimen (BOND). clinicaltrials.gov/ct2/show/NCT03142984 (first received 8 May 2017).
NCT03808532 {published data only}
-
- NCT03808532. Moisturizer to prevent atopic dermatitis (ACE-AD). clinicaltrials.gov/ct2/show/NCT03808532 (first received 17 January 2019).
NCT03871998 {published data only}
-
- NCT03871998. Short-term topical application to prevent atopic dermatitis (STOP AD). clinicaltrials.gov/ct2/show/NCT03871998 (first received 12 March 2019).
NCT04398758 {unpublished data only}
-
- NCT04398758. Moisturizer mediated Prevention of symptoms of Atopic Dermatitis in early childhood (MOPAD). clinicaltrials.gov/ct2/show/NCT04398758 (first received 21 May 2020).
Additional references
Abuabara 2018
Abuabara 2019
ACTRN12613000472774
-
- ACTRN12613000472774. The PEBBLES study: prevention of eczema by a barrier lipid equilibrium strategy [Does twice daily application of a ceramide dominant cream (EpiCeram) for the first six months of life reduce the incidence of eczema by six months of age, when compared to standard skin care, in infants who have a family history of allergic disease]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364115 (first received 26 April 2013).
Amare 2015
Andrews 2013
-
- Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726-35. [DOI: 10.1016/j.jclinepi.2013.02.003] - DOI - PubMed
Apfelbacher 2011
-
- Apfelbacher CJ, Diepgen TL, Schmitt J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy 2011;66(2):206-13. - PubMed
Asai 2020
Asher 2006
-
- Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006;368(9537):733-43. - PubMed
Bai 2017
Barbarot 2018
-
- Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018;73(6):1284-93. - PubMed
Blume‐Peytavi 2016
Bock 1988
-
- Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. Journal of Allergy and Clinical Immunology 1988;82(6):986-97. - PubMed
Botha 2019
Boyce 2010
Boyle 2017
-
- Boyle R, Williams H, Askie L, Lodrup-Carlsen K, Montgomery A, Chalmers J, et al. Prospectively planned meta-analysis of skin barrier studies for the prevention of eczema and associated health conditions. www.crd.york.ac.uk/prospero/display_record.php?RecordID=56965 (first received 10 February 2017).
Carroll 2005
-
- Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatric Dermatology 2005;22(3):192-9. - PubMed
Caussin 2009
-
- Caussin J, Rozema E, Gooris GS, Wiechers JW, Pavel S, Bouwstra JA. Hydrophilic and lipophilic moisturizers have similar penetration profiles but different effects on SC water distribution in vivo. Experimental Dermatology 2009;18(11):954-61. - PubMed
Chalmers 2017
-
- Chalmers JR, Haines RH, Mitchell EJ, Thomas KS, Brown SJ, Ridd M, et al. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (the BEEP trial): protocol for a randomised controlled trial. Trials 2017;18(1):343. - PMC - PubMed
Charman 2004
-
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Archives of Dermatology 2004;140(12):1513-9. - PubMed
Chiou 2004
COMFA 2020
-
- Core Outcome Measures for Food Allergy. www.cost.eu/actions/CA18227/#tabs|Name:overview (accessed prior to 16 September 2020).
Cook 2018
Cork 2002
-
- Cork MJ, Murphy R, Carr J, Buttle D, Ward S, Båvik C, et al. The rising prevalence of atopic eczema and environmental trauma to the skin. Dermatology in Practice 2002;10(3):22-6.
Cro 2020a
-
- Cro S, Boyle R, Kelleher M, Tran L, Cornelius V. Skin care interventions for preventing eczema and food allergy: a statistical analysis plan for a systematic review and individual participant data meta-analysis. zenodo.org/record/3610604#.XiGKU8j7SUk (accessed 16 January 2020). [DOI: 10.5281/zenodo.3610604] - DOI
Cro 2020b
-
- Cro S, Kelleher M, Cornelius V, Boyle R. Skin care interventions for preventing eczema and food allergy: RoB 2 assessments (Version 1) [Data set]. Zenodo. [DOI: ] - PubMed
Danby 2011
-
- Danby SG, Al-Enezi T, Sultan A, Chittock J, Kennedy K, Cork MJ. The effect of aqueous cream BP on the skin barrier in volunteers with a previous history of atopic dermatitis. British Journal of Dermatology 2011;165(2):329-34. - PubMed
Danby 2018
-
- Danby SG, Brown K, Wigley AM, Chittock J, Pyae PK, Flohr C, et al. The effect of water hardness on surfactant deposition after washing and subsequent skin irritation in atopic dermatitis patients and healthy control subjects. Journal of Investigative Dermatology 2018;138(1):68-77. - PubMed
DaSilva 2020
Deckers 2012
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. - PubMed
Drucker 2017
-
- Drucker AM, Wang AR, Li W-Q, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. Journal of Investigative Dermatology 2017;137(1):26-30. - PubMed
Du Toit 2015
Egger 1997
Eichenfield 2014
Eldridge 2016
-
- Eldridge S, Campbell M, Campbell M, Drahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. www.riskofbias.info/welcome/rob-2-0-tool/archive-rob-2-0-cluster-randomi... (accessed 14 October 2020).
Engebretsen 2017
-
- Engebretsen KA, Bager P, Wohlfahrt J, Skov L, Zachariae C, Nybo Andersen AM, et al. Prevalence of atopic dermatitis in infants by domestic water hardness and season of birth: cohort study. Journal of Allergy and Clinical Immunology 2017;139(5):1568-74.e1. - PubMed
Ewence 2011
-
- Ewence A, Rumsby P, Danby S, Cork MJ, Williams HC. A review of skin irritation and tap water. www.dwi.defra.gov.uk/research/completed-research/reports/dwi70-2-257.pdf (accessed prior to 24 January 2019).
Flohr 2010
-
- Flohr C, England K, Radulovic S, McLean WH, Campbell LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. British Journal of Dermatology 2010;163(6):1333-6. - PubMed
Flohr 2014
-
- Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014;69(1):3-16. - PubMed
Furlan 2009
-
- Furlan AD, Pennick V, Bombardier C, Van Tulder M, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929-41. - PubMed
Garcia‐Larsen 2018
Grabenhenrich 2017
Gupta 2004
-
- Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clinical & Experimental Allergy 2004;34(4):520-6. - PubMed
Gupta 2013
-
- Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatrics 2013;167(11):1026-31. - PubMed
Gupta 2019
Hanifin 1980
-
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato-Venereologica 1980;60 Suppl 92:44-7.
Hanifin 2001
-
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Experimental Dermatology 2001;10(1):11-8. - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. - PubMed
Higgins 2003
Higgins 2018
-
- Higgins JP, Savovic J, Page MJ, Sterne JA, ROB2 development group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-versio... (accessed 5 February 2019).
Higgins 2020a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2020b
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Ierodiakonou 2016
-
- Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA 2016;316(11):1181-92. [DOI: ] - PubMed
Irvin 2015
-
- Irvin EJ, Miller HD. Emollient use in the term newborn: a literature review. Neonatal Network 2015;34(4):227-30. - PubMed
Irvine 2011
-
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. New England Journal of Medicine 2011;365(14):1315-27. - PubMed
ISRCTN 21528841
-
- ISRCTN 21528841. Barrier enhancement for eczema prevention. www.isrctn.com/ISRCTN21528841 (first received 25 July 2014).
Jerschow 2014
Johansson 2003
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization. Journal of Allergy and Clinical Immunology 2003;113(5):832-6. - PubMed
JPRN‐UMIN000004544
-
- JPRN-UMIN000004544. Effect of emollients on the prevention of infantile eczema and atopic dermatitis. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000005429 (first received 11 November 2010).
JPRN‐UMIN000010838
-
- JPRN-UMIN000010838. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial, randomized, non-treatment controlled trial. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000012665 (first received 30 May 2013).
JPRN‐UMIN000013260
-
- JPRN-UMIN000013260. Effects of moisturizing skin care from the neonatal stage for improving skin barrier function and preventing skin trouble. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014285 (first received 25 February 2014).
Kelleher 2016
-
- Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. Journal of Allergy and Clinical Immunology 2016;137(4):1111-6.e8. - PubMed
Kelleher 2020b
-
- Kelleher MM, Jay N, Perkin MR, Haines RH, Batt R, Bradshaw LE, et al. An algorithm for diagnosing IgE-mediated food allergy in study participants who do not undergo food challenge. Clinical and Experimental Allergy 2020;50:334-42. - PubMed
Knibb 2010
-
- Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 2010;65:933-45. - PubMed
Leung 2004
Liu 2018
Lodén 2012
-
- Lodén M. Effect of moisturizers on epidermal barrier function. Clinics in Dermatology 2012;30(3):286-96. - PubMed
Luoma 1983
-
- Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of five years. A prospective study of 543 newborns. Allergy 1983;38(5):339-46. - PubMed
Lødrup 2018
-
- Lødrup Carlsen KC, Rehbinder EM, Skjerven HO, Carlsen MH, Fatnes TA, Fugelli P, et al. Preventing atopic dermatitis and allergies in children—the PreventADALL study. Allergy 2018;73(10):2063-70. - PubMed
Mancini 2008
-
- Mancini A, Lawley L. Structure and function of newborn skin. In: Neonatal Dermatology. 2 edition. Philadelphia: Elsevier Saunders, 2008:19-31.
Martin 2013
-
- Martin PE, Koplin JJ, Eckert JK, Lowe AJ, Ponsonby AL, Osborne NJ, et al. The prevalence and socio-demographic risk factors of clinical eczema in infancy: a population-based observational study. Clinical and Experimental Allergy 2013;43(6):642-51. - PubMed
Martin 2015
-
- Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clinical and Experimental Allergy 2015;45(1):255-64. - PubMed
McAleer 2018
-
- McAleer MA, Jakasa I, Raj N, O'Donnell CP, Lane ME, Rawlings AV, et al. Early-life regional and temporal variation in filaggrin-derived natural moisturizing factor, filaggrin-processing enzyme activity, corneocyte phenotypes and plasmin activity: implications for atopic dermatitis. British Journal of Dermatology 2018;179(2):431-41. - PMC - PubMed
Meckfessel 2014
-
- Meckfessel MH, Brandt S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. Journal of the American Academy of Dermatology 2014;71(1):177-84. - PubMed
Munblit 2020
Natsume 2017
-
- Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet 2017;389(10066):276-86. [DOI: 10.1016/S0140-6736(16)31418-0] - DOI - PubMed
NCT01375205
-
- NCT01375205. Comparing cetaphil restoraderm system versus standard skin care in infants. clinicaltrials.gov/ct2/show/results/NCT01375205 (first received 17 June 2011).
NCT02449850
-
- NCT02449850. Preventing atopic dermatitis and allergies in children (PreventADALL). clinicaltrials.gov/ct2/show/NCT02449850 (first received 20 May 2015).
NICE 2006
-
- NICE. Postnatal care up to 8 weeks after birth. www.nice.org.uk/guidance/cg37 (accessed prior to 24 January 2019).
Nikolovski 2008
-
- Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. Journal of Investigative Dermatology 2008;128(7):1728-36. [DOI: 10.1038/sj.jid.5701239] - DOI - PubMed
Oakley 2016
-
- Oakley R, Lawton S. Views on unwanted effects of leave-on emollients and experiences surrounding their incidence. Dermatological Nursing 2016;15(4):38-43.
Osbourne 2011
-
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. Journal of Allergy and Clinical Immunology 2011;127(3):668-76 e1-2. - PubMed
Palmer 2006
-
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics 2006;38(4):441-6. - PubMed
Pawankar 2014
Penzer 2012
-
- Penzer R. Best practice in emollient therapy: a statement for healthcare professionals December 2012. Dermatological Nursing 2012;11(4):60-79.
Perkin 2016
Peters 2018
-
- Peters RL, Koplin JJ, Allen KJ, Lowe AJ, Lodge CJ, Tang ML, et al. The prevalence of food sensitization appears not to have changed between 2 Melbourne cohorts of high-risk infants recruited 15 years apart. Journal of Allergy & Clinical Immunology In Practice 2018;6(2):440-8. [DOI: 10.1016/j.jaip.2017.11.018] - DOI - PubMed
Poulos 2007
-
- Poulos LM, Waters A-M, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. Journal of Allergy and Clinical Immunology 2007;120(4):878-84. - PubMed
Prescott 2013
Ramesh 2017
Rawlings 2004
-
- Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatologic Therapy 2004;17(Suppl 1):49-56. - PubMed
Rendell 2011
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ring 2012
-
- Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. Journal of the European Academy of Dermatology and Venereology 2012;26(8):1045-60. - PubMed
Ronnstad 2018
-
- Rønnstad AT, Halling-Overgaard A-S, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. Journal of the American Academy of Dermatology 2018;79(3):448-56.e30. - PubMed
Rucker 2008
-
- Rucker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Statistics in Medicine 2008;27(5):746-63. - PubMed
Schünemann 2020
-
- Schünemann 20, Higgins JPT, Vist GE, Glasziou et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020.
Sicherer 2003
-
- Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. Journal of Allergy and Clinical Immunology 2003;112(6):1203-7. - PubMed
Simpson 2012
Spergel 2003
-
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. Journal of Allergy and Clinical Immunology 2003;112(6 Suppl):S118-27. - PubMed
Stamatas 2010
Stamatas 2011
-
- Stamatas GN, Nikolovski J, Mack MC, Kollias N. Infant skin physiology and development during the first years of life: a review of recent findings based on in vivo studies. International Journal of Cosmetic Science 2011;33(1):17-24. [PMID: ] - PubMed
Stata [Computer program]
-
- Stata. Version 15. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Stewart 2002
Stewart 2019
-
- Tierney JF, Stewart LA, Clarke M. Chapter 26, Individual patient data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Stone 2002
-
- Stone KD. Atopic diseases of childhood. Current Opinion in Pediatrics 2002;145(5):634-46. - PubMed
Strid 2004
-
- Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. European Journal of Immunology 2004;34(8):2100-9. - PubMed
Strid 2005
-
- Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clinical and Experimental Allergy 2005;35(6):757-66. - PubMed
Su 1997
Surber 2017
-
- Surber C, Kottner J. Skin care products: what do they promise, what do they deliver. Journal of Tissue Viability 2017;26(1):29-36. - PubMed
Taichman 2017
-
- Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, et al. Data sharing statements for clinical trials — a requirement of the International Committee of Medical Journal Editors. New England Journal of Medicine 2017;376(23):2277-9. - PubMed
Thomsen 2015
Tsakok 2016
-
- Tsakok T, Marrs T, Mohsin M, Baron S, du Toit G, Till S, et al. Does atopic dermatitis cause food allergy? A systematic review. Journal of Allergy and Clinical Immunology 2016;137(4):1071-8. - PubMed
Tsilochristou 2019
-
- Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. Journal of Allergy & Clinical Immunology 2019;144(2):494-503. [PMID: ] - PubMed
Turner 2015
-
- Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. Journal of Allergy and Clinical Immunology 2015;135(4):956-63.e1. - PMC - PubMed
Umasunthar 2013
UMIN000028043
-
- UMIN000028043. Early aggressive intervention for infantile atopic dermatitis to prevent development of food allergy: a multicenter, investigator-blinded, randomized, parallel group controlled trial. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031912 (first received 4 July 2017).
Van Cleave 2010
-
- Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA 2010;303(7):623-30. - PubMed
Van den Oord 2009
Van Zuuren 2017
Venter 2008
-
- Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy 2008;63(3):354-9. - PubMed
Visscher 2017
Voorheis 2019
Voskamp 2014
-
- Voskamp AL, Zubrinich CM, Abramovitch JB, Rolland JM, O’Hehir RE. Goat's cheese anaphylaxis after cutaneous sensitization by moisturizer that contained goat's milk. Journal of Allergy and Clinical Immunology 2014;2(5):629-30. - PubMed
Weidinger 2016
-
- Weidinger S, Novak N. Atopic Dermatitis. Lancet 2016;387(10023):1109-22. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. - PubMed
WHO 2015
-
- World Health Organization. Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice. www.ncbi.nlm.nih.gov/books/NBK326678/ (accessed prior to 11 March 2019). - PubMed
Williams 1994
-
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The UK Working Party's diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. British Journal of Dermatology 1994;131(3):383-96. - PubMed
Williams 1998
-
- Williams HC, Strachan DP. The natural history of childhood eczema: observations from the British 1958 birth cohort study. British Journal of Dermatology 1998;139(5):834-9. - PubMed
Woods 2002
-
- Woods RK, Stoney RM, Raven J, Walters EH, Abramson M, Thien FC. Reported adverse food reactions overestimate true food allergy in the community. European Journal Of Clinical Nutrition 2002;56(1):31-6. - PubMed
Yosipovitch 2000
-
- Yosipovitch G, Maayan-Metzger A, Merlob P, Sirota L. Skin barrier properties in different body areas in neonates. Pediatrics 2000;106(1):105-8. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

