Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study
- PMID: 33546093
- PMCID: PMC7913191
- DOI: 10.3390/jcm10040555
Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study
Abstract
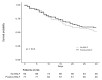
The primary objective of this multicenter, observational, retrospective study was to assess the incidence rate of ventilator-associated pneumonia (VAP) in coronavirus disease 2019 (COVID-19) patients in intensive care units (ICU). The secondary objective was to assess predictors of 30-day case-fatality of VAP. From 15 February to 15 May 2020, 586 COVID-19 patients were admitted to the participating ICU. Of them, 171 developed VAP (29%) and were included in the study. The incidence rate of VAP was of 18 events per 1000 ventilator days (95% confidence intervals [CI] 16-21). Deep respiratory cultures were available and positive in 77/171 patients (45%). The most frequent organisms were Pseudomonas aeruginosa (27/77, 35%) and Staphylococcus aureus (18/77, 23%). The 30-day case-fatality of VAP was 46% (78/171). In multivariable analysis, septic shock at VAP onset (odds ratio [OR] 3.30, 95% CI 1.43-7.61, p = 0.005) and acute respiratory distress syndrome at VAP onset (OR 13.21, 95% CI 3.05-57.26, p < 0.001) were associated with fatality. In conclusion, VAP is frequent in critically ill COVID-19 patients. The related high fatality is likely the sum of the unfavorable prognostic impacts of the underlying viral and the superimposed bacterial diseases.
Keywords: COVID-19; SARS-CoV-2; VAP; coronavirus; ventilation.
Conflict of interest statement
Outside the submitted work, M.B. (Matteo Bassetti) has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, Bayer, Basilea, BioMérieux, Cidara, Gilead, Menarini, MSD, Nabriva, Paratek, Pfizer, Roche, Melinta, Shionogi, Tetraphase, VenatoRx and Vifor and has received study grants from Angelini, Basilea, Astellas, Shionogi, Cidara, Melinta, Gilead, Pfizer and MSD. Outside the submitted work, D.R.G. reports honoraria from Stepstone Pharma GmbH and an unconditional grant from MSD Italia. The other authors have no conflict of interest to disclose.
Figures
References
-
- Bassetti M., Giacobbe D.R., Aliberti S., Barisione E., Centanni S., De Rosa F.G., Di Marco F., Gori A., Granata G., Mikulska M., et al. Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: Current position of the Italian Society of Anti-infective Therapy (SITA) and the Italian Society of Pulmonology (SIP) Clin. Microbiol. Infect. 2020;26:880–894. doi: 10.1016/j.cmi.2020.04.031. - DOI - PMC - PubMed
-
- Grasselli G., Tonetti T., Protti A., Langer T., Girardis M., Bellani G., Laffey J., Carrafiello G., Carsana L., Rizzuto C., et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. - DOI - PMC - PubMed
-
- Robba C., Battaglini D., Ball L., Patroniti N., Loconte M., Brunetti I., Vena A., Giacobbe D.R., Bassetti M., Rocco P.R.M., et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 2020;279:103455. doi: 10.1016/j.resp.2020.103455. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

