Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors
- PMID: 33546102
- PMCID: PMC7913165
- DOI: 10.3390/cancers13040585
Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors
Abstract
Immune checkpoint inhibitors (ICIs) induce durable clinical responses only in a subset of advanced non-small cell lung cancer (NSCLC) patients. There is a need to identify mechanisms of ICI resistance and immunotherapy biomarkers to improve clinical benefit. In this study, we evaluated the prognostic and predictive value of circulating endothelial and leukocyte-derived extracellular vesicles (EV) in patients with advanced NSCLC treated with anti-PD-1/PD-L1 agents. In addition, the relationship between total blood circulating EV proteome and response to ICIs was investigated. An optimized flow cytometry method was employed for the identification and subtyping of blood circulating EVs in 59 patients with advanced NSCLC. Blood samples were collected from patients receiving anti-PD-1/PD-L1 inhibitors (n = 31) or chemotherapy (n = 28). An exploratory proteomic analysis of sorted blood EVs was conducted in a subset of patients. Our results show that a low blood concentration of circulating endothelial-derived EVs before treatment was strongly associated to longer overall survival (p = 0.0004) and higher disease control rate (p = 0.045) in patients treated with ICIs. Interestingly, shotgun proteomics revealed that EVs of responders to anti-PD-1 therapy had a specific protein cargo before treatment. In addition, EV protein cargo was specifically modulated during immunotherapy. We identified a previously unknown association between circulating endothelial-derived extracellular vesicle concentration and immunotherapy-related clinical outcomes. We also observed differences in circulating extracellular vesicle proteome according to anti-PD-1-based treatment response in NSCLC patients. Overall, these results may contribute to the identification of novel circulating biomarkers for rational immunotherapy approaches in patients affected by NSCLC.
Keywords: biomarker; cancer immunotherapy; extracellular vesicles; immune checkpoint inhibitors; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



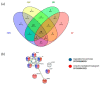
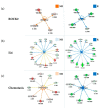
References
-
- Cufaro M.C., Pieragostino D., Lanuti P., Rossi C., Cicalini I., Federici L., De Laurenzi V., Del Boccio P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019;2019:1639854. doi: 10.1155/2019/1639854. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

