COVID-19 Vaccine Acceptance among Health Care Workers in the United States
- PMID: 33546165
- PMCID: PMC7913135
- DOI: 10.3390/vaccines9020119
COVID-19 Vaccine Acceptance among Health Care Workers in the United States
Abstract
Background: Acceptance of the COVID-19 vaccine will play a major role in combating the pandemic. Healthcare workers (HCWs) are among the first group to receive vaccination, so it is important to consider their attitudes about COVID-19 vaccination to better address barriers to widespread vaccination acceptance.
Methods: We conducted a cross sectional study to assess the attitude of HCWs toward COVID-19 vaccination. Data were collected between 7 October and 9 November 2020. We received 4080 responses out of which 3479 were complete responses and were included in the final analysis.
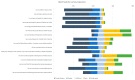
Results: 36% of respondents were willing to take the vaccine as soon as it became available while 56% were not sure or would wait to review more data. Only 8% of HCWs do not plan to get vaccine. Vaccine acceptance increased with increasing age, education, and income level. A smaller percentage of female (31%), Black (19%), Lantinx (30%), and rural (26%) HCWs were willing to take the vaccine as soon as it became available than the overall study population. Direct medical care providers had higher vaccine acceptance (49%). Safety (69%), effectiveness (69%), and speed of development/approval (74%) were noted as the most common concerns regarding COVID-19 vaccination in our survey.
Keywords: Covid-19; United States; healthcare workers; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Johns Hopkins Coronavirus Resource Center Global Map. [(accessed on 6 October 2020)]; Available online: https://coronavirus.jhu.edu/map.html.
-
- Adaptive Phase IB-II Randomized Clinical Trial of Preventive Vaccine Consisting of Autologous Dendritic Cells Loaded with Antigens from Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), with or without GM-CSF, in Subjects Negative for COVID-19 Infection and Anti-SARS-CoV-2 Antibodies. ClinicalTrials.gov identifier (NCT number): NCT04386252. [(accessed on 11 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04386252.
-
- A Randomized, Double-blind, Placebo-controlled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-mediated COVID-19 in Adults Aged 18 Years and Older. ClinicalTrials.gov Identifier: NCT04505722. [(accessed on 11 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04505722.
-
- A Phase 2a, Randomized, Observer-Blind, Placebo Controlled, Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 SARS-COV-2 Vaccine in Adults Aged 18 Years and Older. ClinicalTrials.gov Identifier: NCT04405076. [(accessed on 11 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04405076.
-
- A Phase 1/Phase 2, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Trial to Evaluate the Safety, Tolerability and Immunogenicity of V591 (COVID-19 Vaccine) in Healthy Younger and Older Participants. ClinicalTrials.gov Identifier: NCT04498247. [(accessed on 11 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04498247.
LinkOut - more resources
Full Text Sources
Other Literature Sources