A 3D Bioprinted Material That Recapitulates the Perivascular Bone Marrow Structure for Sustained Hematopoietic and Cancer Models
- PMID: 33546275
- PMCID: PMC7913313
- DOI: 10.3390/polym13040480
A 3D Bioprinted Material That Recapitulates the Perivascular Bone Marrow Structure for Sustained Hematopoietic and Cancer Models
Abstract
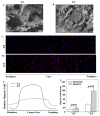
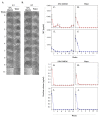
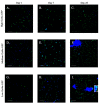
Translational medicine requires facile experimental systems to replicate the dynamic biological systems of diseases. Drug approval continues to lag, partly due to incongruencies in the research pipeline that traditionally involve 2D models, which could be improved with 3D models. The bone marrow (BM) poses challenges to harvest as an intact organ, making it difficult to study disease processes such as breast cancer (BC) survival in BM, and to effective evaluation of drug response in BM. Furthermore, it is a challenge to develop 3D BM structures due to its weak physical properties, and complex hierarchical structure and cellular landscape. To address this, we leveraged 3D bioprinting to create a BM structure with varied methylcellulose (M): alginate (A) ratios. We selected hydrogels containing 4% (w/v) M and 2% (w/v) A, which recapitulates rheological and ultrastructural features of the BM while maintaining stability in culture. This hydrogel sustained the culture of two key primary BM microenvironmental cells found at the perivascular region, mesenchymal stem cells and endothelial cells. More importantly, the scaffold showed evidence of cell autonomous dedifferentiation of BC cells to cancer stem cell properties. This scaffold could be the platform to create BM models for various diseases and also for drug screening.
Keywords: alginate; bioprinting; bone marrow; breast cancer; hydrogel; methylcellulose; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Thomas D.W., Burns J., Audette J., Carroll A., Dow-Hygelund C., Hay M. Clinical Development Success Rates 2006–2015. BIO Biomedtracker Amplion. 2016;1:16.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

