COVID-19 Vaccination: From Interesting Agent to the Patient
- PMID: 33546347
- PMCID: PMC7913564
- DOI: 10.3390/vaccines9020120
COVID-19 Vaccination: From Interesting Agent to the Patient
Abstract
The vaccination for the novel Coronavirus (COVID-19) is undergoing its final stages of analysis and testing. It is an impressive feat under the circumstances that we are on the verge of a potential breakthrough vaccination. This will help reduce the stress for millions of people around the globe, helping to restore worldwide normalcy. In this review, the analysis looks into how the new branch of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) came into the forefront of the world like a pandemic. This review will break down the details of what COVID-19 is, the viral family it belongs to and its background of how this family of viruses alters bodily functions by attacking vital human respiratory organs, the circulatory system, the central nervous system and the gastrointestinal tract. This review also looks at the process a new drug analogue undergoes, from (i) being a promising lead compound to (ii) being released into the market, from the drug development and discovery stage right through to FDA approval and aftermarket research. This review also addresses viable reasoning as to why the SARS-CoV-2 vaccine may have taken much less time than normal in order for it to be released for use.
Keywords: COVID-19; drug development and discovery; pandemic; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); vaccination.
Conflict of interest statement
The author declares no conflict of interest.
Figures
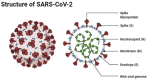


References
-
- Davies N.G., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J., Pearson C.A.B., Russell T.W., Tully D.C., Abbott S., Gimma A., et al. Estimated Transmissibility and Severity of Novel SARS-CoV-2 Variant of Concern 202012/01 in England. [(accessed on 29 December 2020)];2020 Available online: https://cmmid.github.io/topics/covid19/reports/uk-novel-variant/2020_12_....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

