Role of Small GTPase RhoA in DNA Damage Response
- PMID: 33546351
- PMCID: PMC7913530
- DOI: 10.3390/biom11020212
Role of Small GTPase RhoA in DNA Damage Response
Abstract
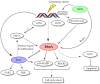
Accumulating evidence has suggested a role of the small GTPase Ras homolog gene family member A (RhoA) in DNA damage response (DDR) in addition to its traditional function of regulating cell morphology. In DDR, 2 key components of DNA repair, ataxia telangiectasia-mutated (ATM) and flap structure-specific endonuclease 1 (FEN1), along with intracellular reactive oxygen species (ROS) have been shown to regulate RhoA activation. In addition, Rho-specific guanine exchange factors (GEFs), neuroepithelial transforming gene 1 (Net1) and epithelial cell transforming sequence 2 (Ect2), have specific functions in DDR, and they also participate in Ras-related C3 botulinum toxin substrate 1 (Rac1)/RhoA interaction, a process which is largely unappreciated yet possibly of significance in DDR. Downstream of RhoA, current evidence has highlighted its role in mediating cell cycle arrest, which is an important step in DNA repair. Unraveling the mechanism by which RhoA modulates DDR may provide more insight into DDR itself and may aid in the future development of cancer therapies.
Keywords: DNA damage response; DNA repair; Ect2; Net1; Rac1; RhoA; cell cycle arrest.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

