Heme Oxygenase-1 Deficiency and Oxidative Stress: A Review of 9 Independent Human Cases and Animal Models
- PMID: 33546372
- PMCID: PMC7913498
- DOI: 10.3390/ijms22041514
Heme Oxygenase-1 Deficiency and Oxidative Stress: A Review of 9 Independent Human Cases and Animal Models
Abstract
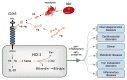
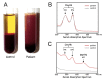
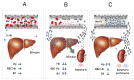
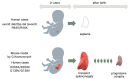
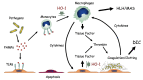
Since Yachie et al. reported the first description of human heme oxygenase (HO)-1 deficiency more than 20 years ago, few additional human cases have been reported in the literature. A detailed analysis of the first human case of HO-1 deficiency revealed that HO-1 is involved in the protection of multiple tissues and organs from oxidative stress and excessive inflammatory reactions, through the release of multiple molecules with anti-oxidative stress and anti-inflammatory functions. HO-1 production is induced in vivo within selected cell types, including renal tubular epithelium, hepatic Kupffer cells, vascular endothelium, and monocytes/macrophages, suggesting that HO-1 plays critical roles in these cells. In vivo and in vitro studies have indicated that impaired HO-1 production results in progressive monocyte dysfunction, unregulated macrophage activation and endothelial cell dysfunction, leading to catastrophic systemic inflammatory response syndrome. Data from reported human cases of HO-1 deficiency and numerous studies using animal models suggest that HO-1 plays critical roles in various clinical settings involving excessive oxidative stress and inflammation. In this regard, therapy to induce HO-1 production by pharmacological intervention represents a promising novel strategy to control inflammatory diseases.
Keywords: HO-1 deficiency; heme oxygenase; inflammation; oxidative stress.
Conflict of interest statement
The author has no conflict interests to declare.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

