Anti-SU Antibody Responses in Client-Owned Cats Following Vaccination against Feline Leukaemia Virus with Two Inactivated Whole-Virus Vaccines (Fel-O-Vax® Lv-K and Fel-O-Vax® 5)
- PMID: 33546485
- PMCID: PMC7913631
- DOI: 10.3390/v13020240
Anti-SU Antibody Responses in Client-Owned Cats Following Vaccination against Feline Leukaemia Virus with Two Inactivated Whole-Virus Vaccines (Fel-O-Vax® Lv-K and Fel-O-Vax® 5)
Abstract
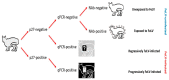
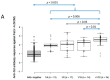
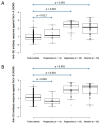
A field study undertaken in Australia compared the antibody responses induced in client-owned cats that had been vaccinated using two inactivated whole feline leukaemia virus (FeLV) vaccines, the monovalent vaccine Fel-O-Vax® Lv-K and the polyvalent vaccine Fel-O-Vax® 5. Serum samples from 428 FeLV-uninfected cats (118 FeLV-vaccinated and 310 FeLV-unvaccinated) were tested for anti-FeLV neutralising antibodies (NAb) using a live virus neutralisation assay to identify 378 FeLV-unexposed (NAb-negative) and 50 FeLV-exposed (NAb-positive; abortive infections) cats, following by anti-surface unit (SU) FeLV-A and FeLV-B antibody ELISA testing. An additional 42 FeLV-infected cats (28 presumptively regressively infected, 14 presumptively progressively infected) were also tested for anti-SU antibodies. NAb-positive cats displayed significantly higher anti-SU antibody ELISA responses compared to NAb-negative cats (p < 0.001). FeLV-unexposed cats (NAb-negative) that had been vaccinated less than 18 months after a previous FeLV vaccination using the monovalent vaccine (Fel-O-Vax® Lv-K) displayed higher anti-SU antibody ELISA responses than a comparable group vaccinated with the polyvalent vaccine (Fel-O-Vax® 5) (p < 0.001 for both anti-FeLV-A and FeLV-B SU antibody responses). This difference in anti-SU antibody responses between cats vaccinated with the monovalent or polyvalent vaccine, however, was not observed in cats that had been naturally exposed to FeLV (NAb-positive) (p = 0.33). It was postulated that vaccination with Fel-O-Vax® 5 primed the humoral response prior to FeLV exposure, such that antibody production increased when the animal was challenged, while vaccination with Fel-O-Vax® Lv-K induced an immediate preparatory antibody response that did not quantitatively increase after FeLV exposure. These results raise questions about the comparable vaccine efficacy of the different FeLV vaccine formulations and correlates of protection.
Keywords: Australia; FeLV infection; FeLV vaccination; humoral immunity; vaccine efficacy; veterinary science.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Shelton G.H., Grant C.K., Cotter S.M., Gardner M.B., Hardy W.D., Jr., DiGiacomo R.F. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: A retrospective study (1968–1988) J. Acquir. Immune. Defic. Syndr. 1990;3:623–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

