Abdominal pain in patients with inflammatory bowel disease: association with single-nucleotide polymorphisms prevalent in irritable bowel syndrome and clinical management
- PMID: 33546600
- PMCID: PMC7866750
- DOI: 10.1186/s12876-021-01622-x
Abdominal pain in patients with inflammatory bowel disease: association with single-nucleotide polymorphisms prevalent in irritable bowel syndrome and clinical management
Abstract
Background: Abdominal pain is a frequent symptom in patients with inflammatory bowel disease (IBD) including Crohn's disease (CD) and ulcerative colitis (UC). Pain can result from ongoing inflammation or functional disorders imitating irritable bowel syndrome (IBS). Several single-nucleotide polymorphisms (SNPs) have been associated with IBS. However, the impact of IBS genetics on the clinical course of IBD, especially pain levels of patients remains unclear.
Methods: Data of 857 UC and 1206 CD patients from the Swiss IBD Cohort Study were analysed. We tested the association of the maximum of the abdominal pain item of disease activity indices in UC and CD over the study period with 16 IBS-associated SNPs, using multivariate ANOVA models.
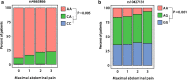
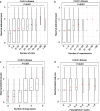
Results: In UC patients, the SNPs rs1042713 (located on the ADRB2 gene) and rs4663866 (close to the HES6 gene) were associated with higher abdominal pain levels (P = 0.044; P = 0.037, respectively). Abdominal pain was not associated with any markers of patient management in a model adjusted for confounders. In CD patients, higher levels of abdominal pain correlated with the number of physician contacts (P < 10-15), examinations (P < 10-12), medical therapies (P = 0.023) and weeks of hospitalisation (P = 0.0013) in a multivariate model.
Conclusions: We detected an association between maximal abdominal pain in UC patients and two IBS-associated SNPs. Abdominal pain levels had a pronounced impact on diagnostic and therapeutic procedures in CD but not in UC patients.
Keywords: Abdominal pain; Crohn’s disease; Inflammatory bowel disease; Irritable bowel syndrome; Single-nucleotide polymorphisms; Ulcerative colitis.
Conflict of interest statement
Martina Ledergerber, Brian M. Lang, Henriette Heinrich, Niklas Krupka, Stefan Begré, Andreas Rickenbacher, Matthias Turina and Niko Beerenwinkel have no competing interests to declare. Luc Biedermann reports grants from the Swiss National Science Foundation, during the conduct of the study; travel grants from Abbvie, MSD, Vifor, personal fees for consulting and advisory board work from Abbvie, Ferring, MSD, Pfizer, Shire, Takeda, UCB, Janssen and a research grant from ThermoFisher, outside the submitted work. Jonas Zeitz received a research grant from Abbvie and has received travel expenses and registration fees for educational events and conferences from Abbvie, Vifor and Almirall, all outside of the submitted work. Thomas Greuter has received travel reimbursements from Vifor and Falk Pharma, personal fees from Sanofi Aventis and a grant from the Novartis Foundation, all for work performed outside the current study. Philipp Schreiner reports travel reimbursements from Vifor, from Pfizer and from UBC, all for work performed outside the submitted work. René Roth reports a personal fee from AbbVie AG, outside the submitted work. Alexander R. Siebenhüner has served at an advisory board and received consulting honoraria from AMGEN, BMS, IPSEN, Lilly, Merck, Pfizer, Sanofi and Servier for work performed outside the current study. Stephan Vavricka has consulted to/received speaker's honoraria from/received research grants from: Abbvie, FALK, Ferring, MSD, Novartis, Pfizer, UCB, Takeda, Tillots, and Vifor, outside the submitted work. Gerhard Rogler has consulted to Abbvie, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; Gerhard Rogler has received speaker's honoraria from Astra Zeneca, Abbvie, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor and Zeller; Gerhard Rogler has received educational grants and research grants from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB and Zeller, for work performed outside the current study. Benjamin Misselwitz has served at an advisory board for Gilead und Novigenix, has received traveling fees or speaking fees from given Imaging, MSD, Vifor, Takeda and Novartis and has received an unrestricted research grant from MSD, outside of the submitted work.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

