Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles
- PMID: 33546702
- PMCID: PMC7866648
- DOI: 10.1186/s12951-021-00776-w
Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles
Abstract
Background: Increasing antibiotic resistance continues to focus on research into the discovery of novel antimicrobial agents. Due to its antimicrobial and wound healing-promoting activity, metal nanoparticles have attracted attention for dermatological applications. This study is designed to investigate the scope and bactericidal potential of zinc ferrite nanoparticles (ZnFe2O4 NPs), and the mechanism of anti-bacterial action along with cytocompatibility, hemocompatibility, and wound healing properties.
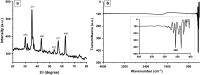
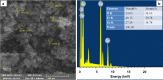
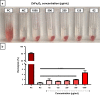
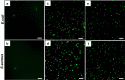
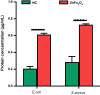
Results: ZnFe2O4 NPs were synthesized via a modified co-precipitation method. Structure, size, morphology, and elemental compositions of ZnFe2O4 NPs were analyzed using X-ray diffraction pattern, Fourier transform infrared spectroscopy, and field emission scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy. In PrestoBlue and live/dead assays, ZnFe2O4 NPs exhibited dose-dependent cytotoxic effects on human dermal fibroblasts. In addition, the hemocompatibility assay revealed that the NPs do not significantly rupture red blood cells up to a dose of 1000 µg/mL. Bacterial live/dead imaging and zone of inhibition analysis demonstrated that ZnFe2O4 NPs showed dose-dependent bactericidal activities in various strains of Gram-negative and Gram-positive bacteria. Interestingly, NPs showed antimicrobial activity through multiple mechanisms, such as cell membrane damage, protein leakage, and reactive oxygen species generation, and were more effective against gram-positive bacteria. Furthermore, in vitro scratch assay revealed that ZnFe2O4 NPs improved cell migration and proliferation of cells, with noticeable shrinkage of the artificial wound model.
Conclusions: This study indicated that ZnFe2O4 NPs have the potential to be used as a future antimicrobial and wound healing drug.
Keywords: Antibiotics; Antimicrobial activity; Biocompatibility; Hemocompatibility; Nanoparticles; Spinel ferrites; Wound healing; Zinc ferrites.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Mohamed H. One year prevalence of critically ill burn wound bacterial infections in surgical ICU in Egypt: Retrospective study. Egyptian Journal of Anaesthesia. 2016;32(3):431–4. doi: 10.1016/j.egja.2016.01.005. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

